Abstract
Transcatheter aortic valve implantation (TAVI) has evolved to a treatment of choice in high-risk patients and is therefore ideal for patients with advanced chronic kidney disease, as patients with end-stage renal disease and kidney transplant recipients. Especially, outcome of this special patient group is very important. 22 patients with chronic kidney disease stage 5 undergoing intermittent hemodialysis treatment (CKD 5D) and 8 kidney transplant recipients (KT) with severe aortic valve stenosis underwent transfemoral TAVI. TAVI was successfully performed in all patients. Postinterventional acute kidney injury (AKI) occurred in four kidney transplant recipients (KDIGO grade 1: n = 3, grade 3: n = 1) but creatinine/eGFR returned to baseline values in all patients. Short-term (30-day) mortality was 3% (1 patient in CKD 5D group). KT had a higher 2-year mortality than CKD5D patients (31% vs. 53%; p = 0.309), and cause of death was non-cardiac because of sepsis in all cases. The amount of contrast medium during TAVI was not associated with the development of acute kidney injury. TAVI is feasible in patients with CKD5D and in KT. Postinterventional AKI in these patients is often mild and does not impact renal function at day 30, while infection/ sepsis is the leading cause of mid-term mortality.
Similar content being viewed by others
Introduction
Transcatheter aortic valve implantation (TAVI) has evolved to a treatment of choice in high-risk patients with symptomatic aortic valve stenosis. Patients with impaired renal function prior to the procedure, especially patients with end-stage renal disease and kidney transplant recipients, represent a special high-risk subgroup.
The outcome of patients with end-stage renal disease and kidney transplant recipients undergoing TAVI is not well-explored but of interest, since TAVI might be a viable alternative to surgical aortic valve replacement for these patients. In the setting of cardiac surgery a decreased estimated glomerular filtration rate (eGFR) is known as a major risk factor for adverse postoperative outcome1, and preoperative chronic hemodialysis reveals high mortality rates after TAVI2. Short-term mortality of kidney transplant recipients undergoing valvular heart surgery is 14%3, while TAVI seems to be an effective method to treat kidney transplant recipients with reduced short-term mortality4. Nevertheless, no data exist on mid-term outcome after TAVI. Besides outcome, a chronic impairment of the kidney transplant is very important. As already stated, acute kidney injury following transcatheter aortic valve implantation (TAVI) is associated with an increased short- (i.e. 30-day) and mid-term mortality5.
Here, we evaluate short-term and mid-term mortality as well as development of postinterventional acute kidney injury in patients with renal replacement therapy and in kidney transplant recipients undergoing transfemoral aortic valve replacement.
Materials and Methods
Patient Population
We retrospectively analyzed data of 30 patients undergoing transfemoral TAVI. 22 patients had chronic kidney disease stage CKD G5 as defined by Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group6 and underwent intermittent hemodialysis treatment prior to the procedure (CKD 5D). 8 patients were kidney transplant recipients (KT).
These patients were part of a consecutive cohort of 710 high-risk or inoperable patients with severe symptomatic aortic valve stenosis who underwent transfemoral TAVI at our center between 2006 and 2016. The local ethics committee of the University of Duisburg-Essen approved this retrospective analysis (No. 16-6894-BO). All procedures were performed in accordance with relevant guidelines and regulations. All patients gave written informed consent for study participation and publication, and the study conformed to the principles of the Declaration of Helsinki.
The indication for TAVI in the individual patient was a consensus decision of the multidisciplinary heart-team (consisting of cardiologists, cardiac surgeons, anesthesiologists and physicians from other disciples whenever needed) according to current guidelines7.
TAVI Procedure
TAVI was performed by a multidisciplinary heart-team in a hybrid operating room using standard techniques8,9, predominantly under conscious sedation10 with percutaneous femoral artery access and closure11. One of two currently CE-approved bioprosthesis (Edwards Sapien and Medtronic CoreValve) was implanted.
All patients were periprocedurally monitored with a 6-electrode virtual 12-lead electrocardiogram and pulse oximetry; an indwelling urinary catheter was inserted. A radial artery catheter and a triple lumen central venous catheter in the internal jugular vein (under ultrasound guidance) were placed, along with a pulmonary artery balloon catheter and a provisional pacemaker catheter10.
All patients were routinely transferred to the intensive care unit (ICU) after the procedure for postinterventional surveillance and further care for a minimum of 24 hours.
Definition acute kidney injury
AKI was defined and staged based on serum creatinine analogous to non-transplant patients according to KDIGO12. Grade 1 is defined as a serum creatinine increase to 1.5–1.9 times baseline or ≥0.3 mg/dl increase within 48 h, grade 2 as an increase in serum creatinine to 2.0–2.9 times baseline, and grade 3 to a serum creatinine increase to 3.0 times baseline or increase to ≥4.0 mg/dl or initiation of renal replacement therapy.
Pre-interventional (24 h before procedur) serum creatinine was defined as baseline. Serum creatinine values were measured daily in all KT for at least 7 days following TAVI.
Statistical Analysis
Data are presented as mean ± standard deviation if normally distributed or as median and interquartile range otherwise. Categorical variables are given as frequencies and percentages. Categorical data were compared between groups using χ2- or Fisher’s exact test. Continuous variables were compared using the Student t-test for dependent and independent samples or the Mann–Whitney U and Wilcoxon signed-rank tests. Kaplan-Meier survival functions were compared with log-rank test. A p-value < 0.05 was considered significant.
Follow-up included data for at least two years following TAVI in each individual patient. All analyses were performed using PASW [SPSS] (Version 21.0, IBM SPSS, Chicago, IL, USA). The authors had full access to the data and take responsibility for their integrity. All authors have read and agreed to the manuscript as written.
Results
Patient characteristics
Our study cohort represents a typical transfemoral TAVI population with severe, symptomatic aortic valve stenosis and high operative risk due to age and comorbidities in addition to renal disease (Table 1). Kidney transplant recipients (KT) were obviously younger (73 ± 4 vs. 79 ± 5 years; p = 0.004) and at lower risk (logistic EuroSCORE 9 ± 5 vs. 27 ± 11%%; p < 0.001) than patients with CKD 5D. Kidney transplant recipients were graded in KDIGO stage CKD 3 T in 3 (38%) and CKD 4 T in 5 cases (62%) at baseline.
Procedure
TAVI was successfully performed in all patients with CKD 5D and in kidney transplant recipients (Table 2). Vascular complications (KT vs. CKD 5D: 13 vs. 18%; p = 0.820), bleeding (25 vs. 14%; p = 0.275) and postprocedural pacemaker implantation (0 vs. 18%; p = 0.208) did not differ between the groups. The different rate in pacemaker implantations can be expected by the fact that the self-expandable bioprosthesis was used in all cases with CKD 5D.
Acute Kidney Injury in Kidney Transplant Recipients
Postinterventional acute kidney injury occurred in four kidney transplant recipients (50%) (Table 3). Acute kidney injury was classified KDIGO grade 1 in three cases, while a single dialysis treatment was performed in one patient (KDIGO grade 3). Serum creatinine had returned to baseline values in all patients at day 30. The amount of contrast agents used was 113 ± 34 ml, and there was no significant difference between the two groups. It was also not associated with the development of acute kidney injury (“AKI” contrast agent: 115 ± 28 ml vs. “no AKI” contrast agent:113 ± 39, p = 0.915). Renal function recovered at day 7 after TAVI procedure (CKD 2 T n = 1; CKD 3 T n = 3; CKD 4 T n = 4).
Mortality
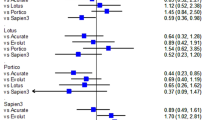
Short-term mortality in this special patient cohort was 3% (one patient in the CKD 5D group). One-year (KT vs. CKD5D: 38% vs. 23%; p = 0.409) and 2-year mortality (KT vs. CKD5D: 53 vs. 31%; p = 0.309) (Fig. 1) was higher in kidney transplant recipients compared to the CKD 5D group. If mortality data is compared to “non-kidney” TAVI patients short-term mortality is lower (KT 0% vs. CKD 5D 3% vs. “non-kidney” 8%; p = 0.596), but 1-year (KT 38% vs. CKD 5D 23% vs. “non-kidney” 19%; p = 0.371) and 2-year mortality (KT 53% vs. CKD 5D 31% vs. “non-kidney” 23%; p = 0.188) are higher in this special patient cohort.
Cause of death
In the period of two years after TAVI, a total of 10 patients died (4 KT and 6 CKD 5D patients). The majority of patients (80%) died due to infections/sepsis (e.g. pneumonia) (Table 4). All 4 deceased kidney transplant recipients died as a result of infections. There were only 2 cardiovascular deaths. One patient (CKD 5D) died at post-interventional day 4 due to systolic heart failure and non-ST elevation myocardial infarction. This patient had a baseline left-ventricular ejection fraction of 11% and was therefore at excessive risk for cardiovascular complications. The other patient died at day 173 due to unknown cause and was therefore classified cardiovascular according to VARC 2 criteria13.
Discussion
This retrospective analysis of single-center data describes short- and mid-term, 2-year outcomes of end-stage renal disease patients and kidney transplant recipients undergoing transfemoral TAVI with focus on the development of postinterventional acute kidney injury and causes of death. Our data revealed that (i) TAVI is feasible and safe with a low short-term mortality in this per se high-risk patient cohort, (ii) the leading cause of death was infection/sepsis during follow-up time and that (iii) acute kidney injury was mild in the majority of cases.
Transfemoral TAVI has meanwhile become a well-established and standardized procedure with low complication rates. Although end-stage renal disease is taken into account in risk scores, kidney transplant recipients are not reflected but apparently need to be considered high-risk patients as well. Hence, we sought to evaluate these two special patient cohorts.
Kidney transplant recipients
Data regarding cardiac survival in kidney transplant recipients are limited and reveal high mortality rates about 20% per year in patients receiving valve replacement3. Therefore, TAVI might be a safe alternative for this rarely investigated cohort4. Yet, TAVI entails certain special risks for kidney transplant recipients. The access site already carries a high level of risk for the transplant, since a local dissection of the pelvic vessels could lead to an impaired blood circulation. The development of AKI, known as an important risk factor for short-term mortality after TAVI in general14,15,16, also compromises transplant function. A postinterventional increase in creatinine with the development of AKI was developed in 50% of the kidney transplant recipients in our study. AKI was mild in 3 of 4 cases and not associated with a higher operative mortality.
In addition, kidney transplant recipients receive immunosuppression therapy, including steroids, mycophenolate and calcineurin inhibitors. Hence, a high short-term mortality might be expected, as TAVI is associated with systemic inflammatory response syndrome (SIRS), which is a strong predictor of mortality17. We did not observe any death within 30 days after procedure. Yet, mid-term mortality rates in kidney transplant recipients were higher than in CKD 5D patients, and, interestingly, all four deceased kidney transplant recipients died as a result of an infection.
CKD 5D patients
Chronic kidney disease (CKD) is an important predictor of mortality after cardiac surgery and has been included in the risk scores in cardiac surgery18,19. Different studies on surgical aortic valve replacement in hemodialysis patients have been performed20,21 clearly demonstrating an increased surgical risk in these patients. Hemodialysis and severe CKD are also strongly associated with increased mortality2,22 in TAVI patients, but early published data are contradictory and only based on small patient numbers2,23. More Recent data revealed a high short- and mid-term mortality in patients with advanced CKD (stage 4 and 5)24.
TAVI intuitively appears as a reasonable option in these high-risk patients. However, they are often judged as too sick even for TAVI. Our study showed a low (5%) short-term mortality, but an unexpectedly high mid-term mortality of 31%. The mid-term mortality is comparable to recent published Italian data by Conrotto et al. (CKD 5D 2-year mortality 56%)24. The higher mid-term mortality of the Italian data could be associated with the higher rate of transapical TAVI (51%)24, which is known to be associated with higher mortality rates in contrast to transfemoral TAVI25,26. Interestingly, the majority of hemodialysis patients died due to infections during the follow-up period. Our experiences show that TAVI is feasible and safe in hemodialysis patients, whereas open surgery is still associated with a substantial rate of mortality up to 20.7% after surgery21. In special cases TAVI was already preferred instead of surgical aortic valve replacement in younger high-risk dialysis patient waitlisted for kidney transplantation due to existing comorbidities27. The uncertainty about the use of TAVI in this population can only be clarified by a dedicated trial.
Limitations
This is a single-center, retrospective observational report with methodology-inherent potential bias that is common for these types of studies. Patients were treated with TAVI over a long time period. Thus, refinements in the TAVI procedure, and also in surgical valves, are not accounted for. Due to this special patient cohort, our study consists of a small number of patients (4.2% of the overall cohort), which only leads to a hypothesis-generating conclusion.
Conclusions
TAVI is feasible and safe in patients with CKD5D and in kidney transplant recipients, who would not be considered candidates for conventional aortic valve replacement due to their high burden of comorbidities. Postinterventional acute kidney injury in these patients is present, but often mild and does not impact renal function at day 30. Infection/Sepsis is the leading cause of mid-term mortality.
References
Mooney, J. F. et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta-analysis. Anesthesiology 118, 809–824 (2013).
Dumonteil, N. et al. Impact of preoperative chronic kidney disease on short- and long-term outcomes after transcatheter aortic valve implantation: a Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC-Plus) initiative substudy. American Heart Journal 165, 752–760 (2013).
Sharma, A., Gilbertson, D. T. & Herzog, C. A. Survival of kidney transplantation patients in the United States after cardiac valve replacement. Circulation 121, 2733–2739 (2010).
Fox, H. et al. Transcatheter aortic valve implantation improves outcome compared to open-heart surgery in kidney transplant recipients requiring aortic valve replacement. J Cardiol 61, 423–427 (2013).
Chen, C. et al. Impact of renal dysfunction on mid-term outcome after transcatheter aortic valve implantation: a systematic review and meta-analysis. PLoS ONE 10, e0119817 (2015).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 1–150 (2013).
Vahanian, A. et al. Guidelines on the management of valvular heart disease (version 2012). European Heart Journal 33, 2451–2496 (2012).
Grube, E. et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving system. Circulation: Cardiovascular Interventions 1, 167–175 (2008).
Webb, J. G. et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 113, 842–850 (2006).
Bergmann, L. et al. Transfemoral aortic valve implantation under sedation and monitored anaesthetic care - a feasibility study. Anaesthesia 66, 977–982 (2011).
Kahlert, P., Eggebrecht, H., Erbel, R. & Sack, S. A modified ‘preclosure’ technique after percutaneous aortic valve replacement. Catheter Cardiovasc Interv 72, 877–884 (2008).
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Supp l 1–138 (2012).
Kappetein, A. P. et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. European Heart Journal 33, 2403–2418 (2012).
Sinning, J. M. et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv 3, 1141–1149 (2010).
Bagur, R. et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. European Heart Journal 31, 865–874 (2010).
Aregger, F. et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol. Dial. Transplant. 24, 2175–2179 (2009).
Sinning, J. M. et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. European Heart Journal 33, 1459–1468 (2012).
Jamieson, W. R. et al. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of The Society of Thoracic Surgeons. The Annals of Thoracic Surgery 67, 943–951 (1999).
Roques, F. et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 15, 816–22– discussion 822–3 (1999).
Tanaka, K. et al. Early and late outcomes of aortic valve replacement in dialysis patients. The Annals of Thoracic Surgery 89, 65–70 (2010).
Herzog, C. A., Ma, J. Z. & Collins, A. J. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation 105, 1336–1341 (2002).
D’Errigo, P. et al. Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement for Severe Aortic Stenosis in Patients With Chronic Kidney Disease Stages 3b to 5. The Annals of Thoracic Surgery 102, 540–547 (2016).
Rau, S. et al. Transcatheter aortic valve implantation in dialysis patients. Nephron Clin Pract 120, c86–90 (2012).
Conrotto, F. et al. Transcatheter Aortic Valve Implantation in Patients With Advanced Chronic Kidney Disease. The American Journal of Cardiology 119, 1438–1442 (2017).
Holmes, D. R. et al. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. The Annals of Thoracic Surgery 101, 789–800 (2016).
Möllmann, H. et al. In-hospital outcome of transcatheter vs. surgical aortic valve replacement in patients with aortic valve stenosis: complete dataset of patients treated in 2013 in Germany. Clinical Research in Cardiology 105, 553–559 (2016).
Büttner, S. et al. Aortic Valve Stenosis in a Dialysis Patient Waitlisted for Kidney Transplantation. The Annals of Thoracic Surgery 102, e437–e438 (2016).
Author information
Authors and Affiliations
Contributions
F.A., A.B. and P.K. initiated the study. F.A., A.B., P.P., H.H., P.K. analyzed the data. F.A., A.B., T.R. and P.K. interpreted the data. The manuscript was written and reviewed by F.A., A.B., H.H., P.P., M.T., A.K., D.W., H.J., A.L., R.A.J., T.R. and P.K. F.A., A.B. and P.K. prepared the figures. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Rashid, F., Bienholz, A., Hildebrandt, H.A. et al. Transfemoral transcatheter aortic valve implantation in patients with end-stage renal disease and kidney transplant recipients. Sci Rep 7, 14397 (2017). https://doi.org/10.1038/s41598-017-14486-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14486-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.