Abstract
Obesity is associated with substantial morbidity, costs, and decreased life expectancy, and continues to rise worldwide. While etiological understanding is needed for prevention, epidemiological studies indicated that colonization with Helicobacter pylori (H. pylori) may affect body mass index (BMI), but with inconsistent results. Here, we examine the relationship between H. pylori colonization and BMI/obesity. Cross-sectional analyses were performed in two independent population-based cohorts of elderly from the Netherlands and Germany (n = 13,044). Genetic risk scores were conducted based on genetic loci associated with either H. pylori colonization or BMI/obesity. We performed a bi-directional Mendelian randomization. Meta-analysis of cross-sectional data revealed no association between anti-H. pylori IgG titer and BMI, nor of H. pylori positivity and BMI. Anti-H. pylori IgG titer was negatively associated with obesity (OR 0.99972; 95% CI 0.99946-0.99997, p = 0.03) and with obesity classes (Beta −6.91 •10−5; 95% CI −1.38•10−4, −5.49•10−7, p = 0.048), but the magnitude of these effects was limited. Mendelian randomization showed no causal relation between H. pylori genetic risk score and BMI/obesity, nor between BMI or obesity genetic risk scores and H. pylori positivity. This study provides no evidence for a clinically relevant association between H. pylori and BMI/obesity.
Similar content being viewed by others
Introduction
The prevalence of obesity rises worldwide. This is associated with significant morbidity, costs, and decreased life expectancy. The latter can be reduced with 8-13 years1, which results in a huge economic burden2. The causes of obesity are diverse and include excessive energy intake, lack of physical activity, but also culprits such as stress, lack of sleep, or exposure to chemical endocrine disruptors3. There is increasing evidence from mouse as well as human studies that shows that the gut microbiome may play an important role in energy balance4. Modern lifestyle, and the widespread use of antibiotics may affect the composition of our microbiome, which may have consequences for our health5.
In this context, Helicobacter pylori (H. pylori), is of relevance. This Gram-negative, spiral-shaped, gastric bacterium is gradually disappearing in Western populations5,6. H. pylori colonization is virtually always associated with chronic active gastritis, which can have various effects. This includes interference with gastric hormone regulation, including ghrelin and leptin. Both have multiple roles in energy homeostasis7. Disturbance of their normal regulation interferes with metabolism and our energy household. H. pylori eradication increases serum ghrelin levels8.
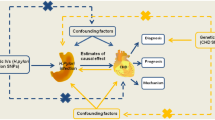
For these reasons, several epidemiological studies have focused on the correlation between H. pylori colonization and BMI and obesity. They showed contrasting results, which were based on H. pylori status and BMI data, but did not include genetic information. A recent genome-wide association study (GWAS) identified two genetic loci associated with anti-H. pylori IgG titers9. Numerous GWAS have identified many genetic loci associated with BMI variation and/or obesity risk10. Combining these results into risk scores enables a Mendelian randomization study for association between H. pylori serology and BMI. Mendelian randomization is a technique that aims at unbiased detection of causal effects11.
We aimed to assess the relationship between H. pylori seroprevalence and obesity using both epidemiological and genetic data of two population-based cohort studies. Results of cross-sectional and genetic analyses were compared. In addition, we performed a meta-analysis of data derived from both cohorts.
Results
Baseline characteristics
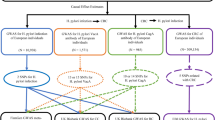
In total, 13,044 participants were initially included in this study. Table 1 summarizes the baseline characteristics of each cohort. In 220 subjects (1.7%) no data on BMI was available. Data on H. pylori titer was lacking in 252 individuals (1.9%) of SHIP. The total population included in the cross-sectional analyses consisted of 12,572 (96.4%) subjects. According to the predefined phenotypic seroprevalence, a total of 3,147 (25.0%) subjects were considered as cases, and 9,425 (75%) as controls.
Genotyping data was available for 6,883 (86.3%) subjects of RS, for 3,824 (93.7%) subjects of SHIP, and for 983 (99.7%) of SHIP-TREND, leaving a total population for analysis of 11,690 (89.6%) subjects. Table 1 summarizes the mean gene risk score for each cohort with respect to the correlation between BMI, obesity, and H. pylori. Linear regression analysis within RS focusing on the BMI gene score and BMI revealed a beta of 0.10 kg/m2 per additional BMI-increasing allele (95% CI 0.07–0.12, p = 5.29•10−16). Logistic regression analysis focusing on the obesity gene score and obesity (BMI ≥ 30 kg/m2) revealed an OR of 1.04 per additional obesity risk allele (95% CI 1.02–1.05, p = 1.09•10−4). The H. pylori gene score was significantly associated with H. pylori positivity (OR 1.39, 95% CI 1.27–1.51; p = 3.46•10−13). The proportion of variance of BMI, obesity, and H. pylori explained by the genetic risk scores ranged from 0.3% for obesity, 0.5% for H. pylori and 1% for BMI per 1 unit increase in score.
Cross-sectional analyses
Cross-sectional analyses revealed an association between H. pylori titer and BMI in RS and SHIP (Supplementary Table S1), however with opposite direction. Meta-analysis of all three cohorts showed no association between H. pylori titer and BMI, nor between H. pylori positivity and BMI (Table 2). H. pylori titer, adjusted for age and sex, was negatively associated with obesity (OR 0.99; 95% CI 0.99–1.00, p = 0.03) and with obesity classes (Beta −6.91 •10−5; 95% CI −1.38•10−4, −5.49•10−7, p = 0.048) (Table 2).
Cross-sectional analyses regarding fecal H. pylori status and BMI/obesity showed no association (Supplementary Table S2).
Mendelian randomization
The BMI gene score was not associated with H. pylori titer or positivity (Table 3). Supplementary Table S3 shows the results of each cohort. Crude analysis showed a positive association between obesity gene score and H. pylori titer (Beta 0.76; 95% CI 0.02–1.50, p = 0.04) (Table 3). H. pylori gene score was not associated with BMI, neither with obesity nor obesity classes (Table 4 and Supplementary Table S4). Also, no associations were observed regarding fecal H. pylori status and the BMI or obesity gene score (Supplementary Table S5).
Discussion
This study included a meta-analysis of 13,044 subjects from two large population-based cohorts. This analysis did not demonstrate an association between H. pylori colonization and BMI, neither when examined by means of serology, nor by fecal antigen, or Mendelian randomization. H. pylori serology, adjusted for age and sex, was negatively associated with obesity (BMI ≥ 30 kg/m2), and obesity classes. However, these effects were small. Active H. pylori colonization, determined by a positive fecal antigen test, was also not positively or negatively associated with obesity. While the unadjusted and adjusted effect estimates for the obesity gene score on anti-H. pylori IgG titer were positive, this association did not remain statistically significant after adjustment for age and sex. So, the use of a Mendelian randomization method did not show a causal bi-directional link between H. pylori serology and BMI or obesity.
Our meta-analysis of H. pylori status as determined by serology showed a small negative association with both obesity and obesity classes. Considering both the small effect estimates, and opposite directions in the individual cohorts, we consider these associations as clinically irrelevant. Prior epidemiological studies have shown either negative12, or positive13,14, or no association15,16 between H. pylori and BMI or obesity. The latter findings are most in line with our findings. A recent review of studies reporting data on H. pylori and obesity prevalence rates in developed countries, showed an inverse correlation (r = −0.29, p < 0.001) between H. pylori colonization and obesity and overweight17. In total, data of 99,463 subjects from 49 studies were pooled. Prevalence rates for H. pylori, but also for overweight and obesity were highly variable between included studies. Nevertheless, no additional analyses were performed to examine whether this correlation was related to potential significant confounders such as age. Age is an important confounder as it is positively correlated with H. pylori colonization18, and negatively with obesity18. This may explain the negative correlation between H. pylori and obesity reported in the systematic review. Other studies have observed weight gain following successful H. pylori eradication19,20,21,22,23. A clinical trial from Japan randomized 1,558 H. pylori-positive adults to either antibiotic treatment or placebo with a subsequent follow-up of 6 months. H. pylori eradication was associated with a mean weight gain of 0.6 kg (95% CI 0.31, 0.88) and an increase in BMI of 0.2 kg/m2 19. The simultaneous improvement of dyspepsia symptoms in the eradication group may have stimulated the appetite and subsequently caused the weight gain by increased food intake. Others have suggested that circulating meal-associated leptin and ghrelin levels, which changed after H. pylori eradication, gave rise to the increased BMI. US investigators observed an increase in both post-prandial levels of leptin and ghrelin a median seven months following H. pylori eradication in a group of 21 patients24. In addition, BMI significantly increased after over 18 months of follow-up, while no change was observed in those who were H. pylori-negative at baseline. Although these studies provided evidence that H. pylori eradication may result in weight gain, it does not imply that there is an absolute difference in BMI between H. pylori-negative and positive subjects. Both our cross-sectional as well as Mendelian randomization results suggest that H. pylori-colonized and H. pylori-negative subjects have similar BMI. The congruence between the results of our cross-sectional and Mendelian randomization analyses is important, as the latter is based on an unbiased approach11.
One of the strengths of this study was the use of different methods to detect H. pylori colonization, by means of both serology and stool antigen. The latter is a reliable method to identify active H. pylori colonization. While most prior studies reported data on serology, we were able to show that results for serology and fecal antigen did not differ. In addition, the use of SNP typing data with genetic risk scores for BMI, obesity, and H. pylori colonization is unique in this field. The Mendelian randomization method is a powerful control for reverse causation and confounding, which otherwise affects epidemiological studies11. It is based on the common disease, common variant hypothesis, which argues that common variants with modest effects underlie many complex traits25.
As any method, the Mendelian randomization has its limitations. Although many genetic variants are discovered, these common variants only explain a small proportion of the estimated trait heritability. Regarding the H. pylori-gene risk score, we were only able to include two genetic variants, due to the fact that no others (as far as we know) have been discovered so far, and these explain only 0.5% of the variance. Similarly, a recent large-scale consortium study estimates that 97 GWAS loci account for ∼2.7% of BMI variation26. Data on H. pylori eradication was not available in the RS and SHIP cohort. Nevertheless, given the selective indications for this both in The Netherlands and in Germany, we can safely assume that this was a small minority of the total population. Finally, we did not account for socio-economic status in the cross-sectional analyses. Although both populations are similar regarding ethnicity and age distribution, differences in socio-economic status are associated with both H. pylori colonization and BMI, and may therefore have influenced our outcome.
In conclusion, this study provides no evidence for a cross-sectional association between H. pylori colonization and BMI or obesity in adults. Mendelian randomization revealed no causal relation between H. pylori and BMI or obesity.
Methods
Study cohorts
The Rotterdam Study is a large, population based prospective study of elderly individuals of European ancestry consisting of three cohorts (RS-I, RS-II, RS-III), who are residing in a suburb of Rotterdam, the Netherlands. The study design has been described in detail previously23,27. Baseline recruitment and measurements for the RS-I study were obtained between 1990 and 1993. The second cohort, RS-II, was set-up in 2000–2001. A third cohort, RS-III, started in 2006 and recruitment ended in December 2008.
The SHIP study comprises two independent prospectively recruited population-based cohorts in Northeastern Germany: SHIP and SHIP-TREND. The study design of SHIP has been described in detail previously18. Participants were recruited between October 1997 and May 2001. SHIP-TREND is an independent cohort from the same region. Individuals were recruited between September 2008 and summer 201218. An important characteristic of SHIP is that it attempts to describe health-related conditions with the widest focus possible.
Data from SHIP, SHIP-TREND, RS-I, and RS-II (RS from now on) were used in this study. Written informed consent was obtained from all participants. Both the medical ethics committee of the Erasmus MC University Medical Center Rotterdam and University Medicine Greifswald approved the study. All methods were performed in accordance with the relevant guidelines and regulations, approved by the medical ethics committee.
Phenotype definition
Serologic H. pylori colonization in individuals from SHIP, SHIP-TREND, and RS was defined by measuring IgG antibody levels in serum using commercial enzyme-linked immunosorbent assay (Pyloriset EIA-G III ELISA; Orion). Seropositivity was defined as an anti-H. pylori IgG titer of ≥20 U/mL according to the manufacturer’s instructions28. Seropositivity is an indicator for current or past colonization. The sensitivity of the Pyloriset EIA-G III ELISA is reported as 97.8%, with a specificity of 58%29. To increase specificity and reduce the number of false-positive H. pylori infections, we defined subjects with the 25% highest IgG titers as H. pylori cases, and those with the 75% lowest IgG titers as controls9.
We further assessed the presence of H. pylori antigen in stool of subjects from SHIP-TREND by using the H. pylori antigen ELISA kit (Immunodiagnostics). For this purpose, 100 mg feces was stored at −20 °C. An optical density (OD) ≥ 0.025 at 450 nm was considered evidence of H. pylori infection, according to the manufacturer’s recommendation. This test has a sensitivity of 97.7% and specificity of 96.3%. H. pylori stool antigen levels and measured anti-H. pylori IgG titers are positively correlated (Spearman ρ = 0.59, p < 0.001)9.
Genetic risk score conduction
For the creation of the genetic risk scores (BMI risk score, obesity risk score, H. pylori risk score), we firstly searched the literature for publications of genome-wide association studies (GWAS) for these traits. A list of SNPs that reached genome-wide significance (P < 5 × 10−8) with BMI or binary obesity status in populations of European ancestry was established. Three different strategies were used to optimize the SNP selection procedure using a key word search (e.g. BMI) on i) the National Human Genome Research Institute (NHGRI) GWAS Catalog (www.genome.gov/gwastudies/) ii) the HuGE Navigator GWAS Integrator (www.hugenavigator.net/HuGENavigator/gWAHitStartPage.do) iii) the PubMed database (www.ncbi.nlm.nih.gov/pubmed). Using this strategy, 45 independent loci were found to be associated with BMI variation and 48 with binary obesity status. We chose to analyze risk scores for BMI and obesity separately. BMI is a phenotype which results in a relatively clean risk score. In contrast, various different definitions have been used to define obesity, like BMI ≥ 25, or BMI ≥ 30. For this reason we used both phenotypes (e.g. BMI continuous and binary) in our analyses. A genotype score (GS) was calculated by summing the alleles of BMI / obesity / H. pylori status-associated SNPs. An unweighted GS was used as previously recommended by Dudbridge et al.30. Imputed genotypes (1000 G PhaseIv3) were used for the creation of the GS. All GWAS published before June 2014 were included. Per genetic locus, 1 SNP was selected based on the following criteria: 1) SNP genotyped with an imputation quality (R2) of 0.3 or higher in all populations; 2) Preference for A/C or G/T variants to avoid strand issues. A list of all studies and variants considered, as well as the variants selected can be found in Supplementary Tables S6, S7 and S8.
Statistical analysis
In total, we used three different approaches to assess the relationship between H. pylori status and BMI/obesity. First, cross-sectional analyses were performed to assess the relationship between H. pylori colonization and BMI/obesity at time of inclusion, by using linear and logistic regression. Outcomes were defined as continuous BMI, binary obesity (BMI ≥ 30 kg/m2, with BMI < 30 kg/m2 as reference group), and obesity classes (lean BMI < 18.5 kg/m2; normal-weight BMI ≥ 18.5 and <25 kg/m2; overweight BMI ≥ 25 and <30 kg/m2; class I obesity BMI ≥ 30 and <35 kg/m2; class II obesity BMI ≥ 35 and <40 kg/m2, class III obesity BMI ≥ 40 kg/m2). The latter outcome was defined as a continuous variable with value ‘0’ for lean, and value ‘5’ for class III obesity. Unadjusted effects of H. pylori titer or antigen (continuous) and H. pylori positivity (binary phenotype) were assessed for each outcome. We additionally adjusted for sex and age. All analyses were done separately for RS, SHIP, and SHIP-TREND. A meta-analysis was performed to observe the combined effect of H. pylori colonization on BMI/obesity.
Second, a Mendelian randomization approach was carried out to explore a bi-directional link between H. pylori colonization and BMI. Linear regression analysis was used to assess the relationship between BMI gene score and H. pylori titer and H. pylori positivity. Analyses were first adjusted for sex, age, and additionally for BMI at baseline. The same analyses were done regarding the obesity gene score. Linear regression analysis was also used to examine the effect of the H. pylori gene score with BMI, binary obesity status, and obesity classes, all defined at baseline. Analyses were adjusted for sex, and age, and additionally for H. pylori status (binary phenotype). Data of the three cohorts were combined and examined by using meta-analysis approach. A p-value < 0.05 was considered to be statistically significant.
All measures of associations are presented as Odds Ratios (OR) or Beta’s with their 95% confidence intervals (CI). Statistical analyses were performed using IBM SPSS Statistics 21.0 for Windows (SPSS IBM, Armonk, New York, USA). Meta-analyses were done with R library meta (R Core Team (2014), R Foundation for Statistical Computing, Vienna, Austria).
Data availability
Due to ethical restrictions data are available upon request. Interested researchers may contact our data management team (secretariat.epi@erasmusmc.nl) for access to sensitive data.
References
Fontaine, K. R., Redden, D. T., Wang, C., Westfall, A. O. & Allison, D. B. Years of life lost due to obesity. JAMA 289, 187–193 (2003).
Finkelstein, E. A., Trogdon, J. G., Cohen, J. W. & Dietz, W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 28, w822–831 (2009).
McAllister, E. J. et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 49, 868–913 (2009).
DiBaise, J. K. et al. Gut microbiota and its possible relationship with obesity. Mayo Clinic proceedings 83, 460–469 (2008).
Blaser, M. J. & Falkow, S. What are the consequences of the disappearing human microbiota? Nature reviews. Microbiology 7, 887–894 (2009).
den Hollander, W. J. et al. Intergenerational reduction in Helicobacter pylori prevalence is similar between different ethnic groups living in a Western city. Gut 64, 1200–1208 (2015).
Klok, M. D., Jakobsdottir, S. & Drent, M. L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity reviews: an official journal of the International Association for the Study of Obesity 8, 21–34 (2007).
Nwokolo, C. U., Freshwater, D. A., O’Hare, P. & Randeva, H. S. Plasma ghrelin following cure of Helicobacter pylori. Gut 52, 637–640 (2003).
Mayerle, J. et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 309, 1912–1920 (2013).
Pigeyre, M., Yazdi, F. T., Kaur, Y. & Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 130, 943–986 (2016).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human molecular genetics 23, R89–98 (2014).
Vo, H. D. et al. Inverse Correlation Between Helicobacter pylori Colonization and Obesity in a Cohort of Inner City Children. Helicobacter 20, 64–68 (2015).
Chen, T. P. et al. Helicobacter Pylori Infection is Positively Associated with Metabolic Syndrome in Taiwanese Adults: a Cross-Sectional Study. Helicobacter 20, 184–191 (2015).
Xu, C. et al. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter 19, 437–442 (2014).
Cho, I. et al. Helicobacter pylori and overweight status in the United States: data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 162, 579–584 (2005).
Ioannou, G. N., Weiss, N. S. & Kearney, D. J. Is Helicobacter pylori seropositivity related to body mass index in the United States? Aliment Pharmacol Ther 21, 765–772 (2005).
Lender, N. et al. Review article: Associations between Helicobacter pylori and obesity–an ecological study. Aliment Pharmacol Ther 40, 24–31 (2014).
Volzke, H. et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol 40, 294–307 (2011).
Lane, J. A. et al. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther 33, 922–929 (2011).
Jang, E. J. et al. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol 23(Suppl 2), S278–285 (2008).
Yang, Y. J., Sheu, B. S., Chang, W. L., Cheng, H. C. & Yang, H. B. Increased body mass index after H. pylori eradication for duodenal ulcer predisposes to erosive reflux esophagitis. J Clin Gastroenterol 43, 705–710 (2009).
Suto, H. et al. The effects of Helicobacter pylori eradication on body mass index and dyspeptic symptoms. Digestion 79, 235–242 (2009).
Hofman, A. et al. The Rotterdam Study: objectives and design update. Eur J Epidemiol 22, 819–829 (2007).
Francois, F. et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol 11, 37 (2011).
Schork, N. J., Murray, S. S., Frazer, K. A. & Topol, E. J. Common vs. rare allele hypotheses for complex diseases. Current opinion in genetics & development 19, 212–219 (2009).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Hofman, A. et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 30, 661–708 (2015).
Rehnberg-Laiho, L. et al. Accelerated decline in Helicobacter pylori seroprevalence rate during the screen and treat project in Vammala, Finland, as demonstrated in 29- to 45-year-old pregnant women. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 112, 34–38 (2004).
Hanvivatvong, O., Pongpanich, A., Thong-Ngam, D., Thammacharoenrach, N. & Kullavanijaya, P. Evaluation of commercial immunoassays for detection of antibody against Helicobacter pylori in Thai dyspeptic patients. Clinical and diagnostic laboratory immunology 11, 618–620 (2004).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS genetics 9, e1003348 (2013).
Acknowledgements
The Rotterdam Study is supported by the Erasmus MC University Medical Center and Erasmus University Rotterdam; The Netherlands Organisation for Scientific Research (NWO); The Netherlands Organisation for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); The Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. The SHIP project is part of the community medicine research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research, the Ministry of Cultural Affairs, as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. None of the funding organizations were involved in study design, in collection, analysis, and interpretation of data, in writing of the report, or in the decision to submit the article for publication. DM holds a Canada Research Chair in Genetics of Obesity.
Author information
Authors and Affiliations
Contributions
W.H., L.B., C.S., D.M., A.U., M.L., E.K. contributed to the conception and design, acquisition of data, analyses and interpretation of the data, drafted the article, revised it critically for important intellectual content and gave final approval of the version to be published. C.H., J.M., A.H., G.H. contributed to the conception and design and acquisition of data, revised the article critically for important intellectual content and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
den Hollander, W.J., Broer, L., Schurmann, C. et al. Helicobacter pylori colonization and obesity – a Mendelian randomization study. Sci Rep 7, 14467 (2017). https://doi.org/10.1038/s41598-017-14106-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14106-4
This article is cited by
-
The association between Helicobacter pylori and obesity: a systematic review and meta-analysis of case–control studies
Clinical Diabetes and Endocrinology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.