Abstract
Plant GDP-D-mannose epimerase (GME) converts GDP-D-mannose to GDP-L-galactose, a precursor of both L-ascorbate (vitamin C) and cell wall polysaccharides. However, the genetic functions of GME in Arabidopsis are unclear. In this study, we found that mutations in Arabidopsis GME affect pollen germination, pollen tube elongation, and transmission and development of the male gametophyte through analysis of the heterozygous GME/gme plants and the homozygous gme plants. Arabidopsis gme mutants also exhibit severe growth defects and early leaf senescence. Surprisingly, the defects in male gametophyte in the gme plants are not restored by L-ascorbate, boric acid or GDP-L-galactose, though boric acid rescues the growth defects of the mutants, indicating that GME may regulate male gametophyte development independent of L-ascorbate and GDP-L-galactose. These results reveal key roles for Arabidopsis GME in reproductive development, vegetative growth and leaf senescence, and suggest that GME regulates plant growth and controls male gametophyte development in different manners.
Similar content being viewed by others
Introduction
L-Ascorbate (vitamin C), a common natural water-soluble antioxidant in plants1, affects plant growth2, 3, leaf senescence4 and photosynthesis5, and it regulates plant responses to pathogen infection6 and various abiotic stresses7,8,9,10. L-Ascorbate is mainly biosynthesised from D-glucose via sequential enzymatic reactions in the L-galactose (L-Gal) pathway11, 12. GDP-D-mannose epimerase (GME) catalyses the conversion of GDP-D-mannose to GDP-L-Gal and GDP-L-gulose, which is a key step in the L-ascorbate pathway13,14,15. In addition, GDP-L-Gal acts as a precursor of cell wall polysaccharides such as rhamnogalacturonan II (RGII), which is a crucial polysaccharide component of pectin16, 17.
GME is the most conserved gene in the ascorbate biosynthesis pathway18. Tomato contains two homologous GMEs19, while most plants such as Arabidopsis 13, rice20, Medicago 21 and peach22 have only one copy of GME 20. In this study, we isolated Arabidopsis T-DNA insertion mutants of GME to examine its biological functions. We found that Arabidopsis GME is vital for plant vegetative growth, leaf senescence and male gametophyte development and transmission.
Results
The expression pattern and subcellular localisation of Arabidopsis GME
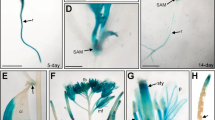
We generated transgenic plants harbouring the GUS reporter driven by the GME promoter (P GME ::GUS) to analyse the GME expression pattern in Arabidopsis. GUS activity was detected in roots, leaves, stems and inflorescences, implying that GME plays key roles in growth and development (Fig. 1A,B,F and G). Moreover, the floral organs of the plants (sepals, petals, stamens and carpels), including pollen grains and pollen tubes, exhibited GUS activity, suggesting that GME affects reproductive development (Fig. 1C–E and H–J). We also examined the GME expression pattern by reverse transcription PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR), and found that the GME expression level was high in rosette leaves, stem leaves and flowers, and low in roots and stems (Fig. 1K and L). These results indicate that GME may affect various aspects of plant growth and development.
Expression pattern and subcellular localisation of GME in Arabidopsis. (A–J) Histochemical GUS activity in seedlings (A), inflorescences (B), flowers (C), pollen grains (D) and pollen tubes (E) from T1 transgenic Arabidopsis expressing the GUS reporter gene under the control of the GME promoter (P GME ::GUS) with GUS activity in wild type (I,J) as a control. (K) and (L) RT-PCR analysis (K) and real-time PCR analysis (L) of the GME expression level in Arabidopsis roots (R), stems (S), stem leaves (SL), rosette leaves (RL) and flowers (F). ACTIN2 was used as the normalisation or internal control. (M) Subcellular localisation of GME in epidermal cells from N. benthamiana leaves. GME-fused GFP driven by the 35S promoter (35S::GME-GFP) and 35S::GFP were expressed in N. benthamiana leaves, respectively. GFP fluorescence was detected 50 h after infiltration. Infiltration with buffer was used as a negative control. BF, bright field. (N) Arabidopsis protoplasts transformed without (Control) or with 35S::GME-GFP. Red fluorescence indicates chloroplasts.
We next transiently expressed GME fused with GFP (GME-GFP) in Nicotiana benthamiana leaves to observe the subcellular localisation of GME. Strong GFP fluorescence was detected in the cytoplasm of epidermal cells (Fig. 1M); fluorescence due to GFP alone (as a control) was detected in both the cytoplasm and nucleus (Fig. 1M). To confirm the subcellular localisation of GME, we transiently expressed GME-GFP in Arabidopsis protoplasts and observed GFP fluorescence only in the cytoplasm (Fig. 1N). These results demonstrate that GME is localised in the cytoplasm (Fig. 1M and N).
Arabidopsis GME controls male gametophyte transmission
We next examined two T-DNA insertion mutants of GME: gme-1 (CS827235, with a T-DNA insertion in the last exon of GME) and gme-2 (Salk_008960, with a T-DNA insertion in the 5′-untranslated region [UTR] of GME) (Fig. 2A and Supplemental Fig. 1). Interestingly, we obtained gme-2 homozygotes, but not gme-1 homozygotes. The progeny of the GME/gme-1 heterozygotes included only wild-type (WT) plants and GME/gme-1 heterozygotes, and the siliques of GME/gme-1 appeared normal without any aborted seeds (Supplemental Fig. 2), implying that the T-DNA insertion in gme-1 attenuated transmission of the male gametophyte, but not embryonic development.
Characterisation and genetic complementation of the gme-1 mutation and an analysis of tricellular pollen grains. (A) Schematic diagram showing the T-DNA insertion in GME and the core structure of the P GME ::GME vector (GME under the control of the GME promoter). The grey rectangle, black rectangle and triangle represent the UTR, exon and T-DNA insertion site, respectively. The primers indicated by arrows were used to identify the genetic background of the gme-1 mutants in (B). (B) Genotyping of GME/gme-1, Col-0 wild type (WT), and transgenic P GME ::GME in a gme-1 background. gme-1 P GME ::GME was generated by transforming P GME ::GME into GME/gme-1 heterozygous plants. The primer pairs LP1/SailLB3 and LP1/RP1, respectively, are specific for the gme-1 T-DNA insertion and WT GME. ACTIN2 PCR products were used as a control. (C) Six-week-old Col-0 WT and gme-1 P GME ::GME plants. (D) Environmental scanning electron microscopy of pollen grains at floral stage 13 from WT, GME/gme-1 and gme-2 plants. Bars = 20 µm. (E) The gme-1 mutation does not affect male meiosis, mitosis, pollen viability or pollen vacuole development at the tricellular stage. The panels from left to right show tetrad pollen grains at the tricellular stage stained, respectively, with DAPI, Alexander’s stain, fluorescein diacetate/propidium iodide and neutral red. Bars = 20 µm.
We next performed reciprocal crosses to determine which type of gametophyte development was affected in GME/gme-1. When the stigmas of GME/gme-1 heterozygotes were pollinated with WT pollen grains, the progeny segregation ratio (GME/GME:GME/gme-1) was identical to the expected 1:1 (Table 1). Conversely, when pollen grains from GME/gme-1 heterozygotes were crossed onto WT stigmas, all progeny were wild type (Table 1), suggesting that gme-1 is a null mutation that leads to complete failure of transmission of the male gametophyte, but not the female gametophyte. We also transformed GME/gme-1 heterozygotes with the coding sequence of GME driven by its native promoter (P GME ::GME) to obtain P GME ::GME transgenic plants in a gme-1 background (gme-1 P GME ::GME) (Fig. 2B and C), demonstrating that GME complemented the transmission defect caused by the gme-1 mutation.
In reciprocal crosses performed with GME/gme-2, when the stigmas of GME/gme-2 heterozygotes were pollinated with WT pollen grains, the progeny exhibited a 1:1 segregation ratio (GME/GME:GME/gme-2) (Table 1). However, when pollen grains from GME/gme-2 plants were applied to WT stigmas, the progeny segregation ratio (GME/GME:GME/gme-2) was about 1:0.12 (Table 1), suggesting that gme-2 is a strong mutation that dramatically reduces the transmission efficiency of the male gametophyte, but that it’s not a null mutation. Consistent with this conclusion, the progeny segregation ratio (gme-2/gme-2 and GME/gme-2:GME/GME) from GME/gme-2 plants was about 1.25:1, which is a clear reduction compared with a normal 3:1 segregation ratio (Table 1). We obtained only 15 gme-2 homozygotes from 182 progeny of a GME/gme-2 heterozygote (8.2%), which is much less than expected (25%).
Taken together (Table 1, Fig. 2A–C and Supplemental Figs 1 and 2), these results show that Arabidopsis GME is essential for male gametophyte transmission.
GME is required for pollen germination and pollen tube elongation
In Arabidopsis anthers, the microspore mother cells undergo sequential meiosis and mitosis to form tricellular pollen grains containing two sperm cell nuclei and one vegetative cell nucleus. Pollen grains from dehisced anthers are released onto the stigma of a carpel and germinate to form pollen tubes, which elongate and go through the stigma and transmitting tract, thereby delivering the two sperm cells to an ovary for double fertilisation23,24,25,26. Any disruption in this process will lead to failed male gametophyte transmission.
We next explored in which stages GME regulates male gametophyte development. Observation of the surface of mature pollen grains from WT, GME/gme-1 heterozygous and gme-2 homozygous plants by environmental scanning electron microscopy showed that all of the pollen grains from the gme mutants were oval-shaped with long indented lines on their surface as in wild type (Fig. 2D).
We next crossed GME/gme-1 plants with the quartet1 (qrt) mutant, which releases unseparated pollen tetrads derived from a single pollen mother cell27, to generate qrt/qrt GME/gme-1 plants. 4′,6-Diamidino-2-phenylindole (DAPI) staining showed that all four pollen grains of the qrt/qrt GME/gme-1 tetrads contained three nuclei (Fig. 2E). Alexander staining, fluorescein diacetate/propidium iodide double staining, and neutral red staining showed that all of the pollen grains from qrt/qrt GME/gme-1 were viable with normal pollen vacuoles (Fig. 2E). These results (Fig. 2E) demonstrate that GME does not affect the formation of tricellular pollen grains.
In vitro pollen germination assays were performed to explore whether the processes that occur after tricellular pollen grain formation are affected in gme mutants. As shown in Fig. 3A and B, the pollen grains from gme-1 heterozygous and gme-2 homozygous plants exhibited reduced germination rates (~39% for GME/gme-1 and ~65% for gme-2) compared with wild type (~80%). Consistently, a maximum of four pollen grains from the qrt/qrt pollen tetrads could germinate, while no more than two pollen grains from the qrt/qrt GME/gme-1 pollen tetrads could germinate (Fig. 3D and E). Thus, GME is required for pollen germination.
GME is required for pollen germination and pollen tube elongation. (A–C) In vitro pollen germination (A), statistical analysis of the pollen germination rate (B) and pollen tube length (C) in Col-0 WT, GME/gme-1, gme-2, gme-2 P GME ::GME and gme-1/gme-2 P GME ::GME plants. Error bars represent the standard error (SE; n = 3). Asterisks represent Student’s t-test significance compared with wild type (*P < 0.05, **P < 0.01). (D) In vitro pollen germination in qrt/qrt and qrt/qrt GME/gme-1. (E) Frequency (%) of qrt/qrt and qrt/qrt GME/gme-1 tetrads with the indicated numbers (0, 1, 2, 3 or 4) of germinated pollen grains. Error bars represent the SE (n = 3). Asterisks represent Student’s t-test significance between pairs indicated with brackets (*P < 0.05, **P < 0.01). (F–H) Aniline blue staining of pollen tubes showing that WT (F) and gme-2 P GME ::GME (H) pollen tubes germinated for 16 h in WT female organs could reach the base of the transmitting tract, while gme-2 pollen tubes (G) could only reach half of the transmitting tract. Consistently, mature WT (F) and gme-2 P GME ::GME (H) siliques were full of seeds, while gme-2 siliques (G) contained few seeds in the upper part of the silique. White arrows indicate pollen tubes.
Moreover, the pollen tube length in gme-2 homozygous plants was much shorter than that in wild type (Fig. 3A and C), indicating that GME is required for pollen tube elongation. To confirm this conclusion, we performed in vivo pollen tube growth experiments. WT pollen tubes could reach the bottom of the transmitting tract (Fig. 3F), and WT siliques were consistently filled with seeds (Fig. 3F). In contrast, the pollen tubes of gme-2 plants could reach no more than half the length of the transmitting tract, and mature gme-2 siliques possessed few seeds that were mainly located in the upper part of the siliques (Fig. 3G).
Taken together (Fig. 3), these results demonstrate that GME is required for pollen germination and pollen tube elongation. In support of this conclusion, genetic complementation experiments showed that GME with its native promoter (P GME ::GME) could rescue in vitro pollen germination, pollen tube elongation, in vivo pollen tube elongation and seed setting in gme-2 plants and in gme-1/gme-2, which was generated by crossing GME/gme-1 with gme-2 (Fig. 3A–C and H).
Defective male gametophyte development in gme mutant plants is not due to an ascorbate deficiency
As GME is a key enzyme in L-ascorbate biosynthesis, we next explored whether the defects in male gametophyte development in the gme mutant plants was due to a deficiency in L-ascorbate. qRT-PCR analysis confirmed that GME expression was reduced in GME/gme-1 and gme-2 plants (Fig. 4A) and decreased in gme-2 pollen grains (Fig. 4B). The ascorbate contents in GME/gme-1 and gme-2 plants were reduced to 64% and 28% of the WT level, respectively (Fig. 4B), demonstrating that ascorbate biosynthesis was decreased in these Arabidopsis gme mutants.
ASA or NaSA application cannot rescue the fertility of gme-2. (A) Real-time PCR analysis of the GME expression levels in WT, GME/gme-1 heterozygous and gme-2 homozygous plants. ACTIN2 was used as the internal control. Error bars represent the SE (n = 3). Asterisks represent Student’s t-test significance compared with wild type (**P < 0.01). (B) Real-time PCR analysis of GME in pollen grains at floral stage 13 from WT and gme-2 homozygous plants. (C) Total ascorbate contents in leaves from 5-week-old WT, GME/gme-1 and gme-2 plants. FW, fresh weight. Error bars represent the SE (n = 3). (D) Inflorescences from 6-week-old WT and gme-2 plants were treated with mock, 1 mM ASA or 1 mM NaSA for 10 days, and the derived siliques were imaged. (E) In vitro pollen germination of qrt/qrt and qrt/qrt GME/gme-1 treated with mock or 50 µM ASA. (F) Pollen germination rates in qrt/qrt and qrt/qrt GME/gme-1 treated with 0, 10, 50, 100, 500 or 1000 µM ASA, respectively. Error bars represent the SE (n = 3). (G) The maximum number of germinated pollen grains per tetrad of qrt/qrt and qrt/qrt GME/gme-1 in (E).
We next investigated whether the application of ascorbate could restore male gametophyte development in gme plants. The inflorescences of WT and gme-2 plants were treated with L-ascorbic acid (ASA) and sodium ascorbate (NaSA), respectively. WT plants exhibited good fertility when treated without or with ASA or NaSA, while male fertility in gme-2 could not be rescued by ASA or NaSA (Fig. 4D). Thus, ascorbate application could not rescue male gametophyte development in gme-2.
In vitro pollen germination assays were performed to detect whether ascorbate could rescue pollen germination in qrt/qrt GME/gme-1. As shown in Fig. 4E–G, ASA application could not recover the pollen germination rate in GME/gme-1, and it even inhibited pollen germination at high concentrations. Similar results were obtained for NaSA (data not shown). These results indicate that the defects in male gametophyte development in gme plants cannot be attributed to an ascorbate deficiency.
We next analysed mutants of the ascorbate biosynthetic genes VTC2 and VTC5, which encode two GDP-L-Gal phosphorylases that function redundantly to control Arabidopsis ascorbate biosynthesis2, in order to verify that an ascorbate deficiency does not affect male gametophyte development. The double mutant vtc2 vtc5 contained only ~22% of the WT level of ascorbate, and it exhibited severe growth defects (Supplemental Fig. 3), demonstrating that ascorbate biosynthesis in vtc2 vtc5 was severely blocked. We also used vtc2/vtc2 VTC5/vtc5 and vtc5/vtc5 VTC2/vtc2 plants that were homozygous for one allele and heterozygous for the other to perform reciprocal crosses with wild type. As shown in Supplemental Table 1, regardless of whether WT plants or mutants were used as recipients, gametophyte transmission was unaffected, suggesting that the abolishment of ascorbate biosynthesis by the mutation of both vtc2 and vtc5 does not affect male gametophyte development.
Taken together (Fig. 4 and Supplemental Table 1), our data demonstrate that an ascorbate deficiency is not responsible for the defects in male gametophyte development observed in gme mutant plants.
Boric acid and GDP-L-Gal cannot restore pollen germination and pollen tube growth in gme mutant plants
A previous study showed that the growth defects in GME-silenced tomato plants could be rescued by the application of boric acid, which promotes the boron-mediated in muro cross-linking of cell wall polysaccharides, but not by ascorbate28. We thus explored whether boric acid could rescue male gametophyte development in our gme mutant plants. In vitro pollen germination assays using qrt/qrt and qrt/qrt GME/gme-1 supplied with different concentrations of boric acid showed that qrt/qrt displayed high pollen germination rates (~65–67%), while qrt/qrt GME/gme-1 treated with different concentrations of boric acid exhibited low germination rates (~31–33%) (Supplemental Fig. 4A and B). These findings suggest that boric acid cannot restore pollen germination in qrt/qrt GME/gme-1.
Next, inflorescences from WT and gme-2 plants were treated with boric acid. Regardless of whether they were treated with or without boric acid, the WT siliques were large and full of seeds while gme-2 produced small siliques with few seeds (Supplemental Fig. 4C), indicating that boric acid supplementation could not restore male gametophyte development and fertility in gme-2.
As GDP-L-Gal is a precursor of cell wall polysaccharides (e.g., RGII)19, we also tested whether the application of GDP-L-Gal could restore pollen germination in GME/gme-1. Our results indicate that GDP-L-Gal was unable to recover pollen germination in qrt/qrt GME/gme-1 (Supplemental Fig. 4D).
Taken together, these data (Supplemental Fig. 4) demonstrate that treatment with boric acid or GDP-L-Gal cannot restore male gametophyte development in gme mutants.
Growth defects in gme mutants
We next investigated whether Arabidopsis GME regulates growth. As shown in Fig. 5, gme-2 homozygous plants exhibited retarded growth in terms of their rosette leaves, height, stem diameter and fertility (e.g., silique length and seed number per silique; Fig. 5). Compared with gme-2, gme-1/gme-2 plants showed much more severe growth defects, including a dramatically reduced rosette leaf size, thinner stems, shorter siliques and fewer seeds (Fig. 5 and Supplemental Fig. 1). The growth defects of gme-2 and gme-1/gme-2 could be restored by genetic complementation with GME (Fig. 5). Thus, GME plays important roles in vegetative growth.
Growth defects and genetic complementation of the gme-2 and gme-1/gme-2 mutants. (A) Morphology of 6-week-old WT, gme-2, gme-1/gme-2, gme-2 P GME ::GME and gme-1/gme-2 P GME ::GME plants. (B–F) Histograms showing the seed number per silique (B), silique length (C), height (D), rosette leaf diameter (E) and stem diameter (F) of the plants in (A). Asterisks represent Student’s t-test significance compared with wild type (*P < 0.05, **P < 0.01).
The growth defects in gme can be rescued by boric acid but not ascorbate
To explore the reason for the growth defects of the Arabidopsis gme mutants, we treated gme-1/gme-2 plants with boric acid, ASA or L-Gal, respectively. As shown in Fig. 6A and B, the growth defects of the gme-1/gme-2 mutant could be rescued by boric acid, but not by ASA or L-Gal, suggesting that the growth defects of the gme-1/gme-2 mutant were due to reduced in muro cross-linking of cell wall polysaccharides. On the other hand, the severe growth defects of the ascorbate-deficient mutant vtc2 vtc5 could be recovered by ASA and L-Gal, but not by boric acid (Supplemental Fig. 5), suggesting that the growth defects of the vtc2 vtc5 double mutant were due to an ascorbate deficiency rather than in muro cross-linking of cell wall polysaccharides.
The growth defects of gme-1/gme-2 can be rescued by boric acid. (A) Three-week-old WT and gme-1/gme-2 seedlings grown on boric acid-free MS medium supplied without (Mock) or with 100 µM boric acid, 100 µM ASA or 100 µM L-Gal. (B) Rosette leaf diameter of the seedlings shown in (A). Error bars represent the SE (n = 8). Asterisks represent Student’s t-test significance compared with wild type (**P < 0.01).
In conclusion, these results (Fig. 6 and Supplemental Fig. 5) demonstrate that the growth defects of gme mutants are caused by reduced in muro cross-linking of cell wall polysaccharides.
Early leaf senescence in gme mutants
Further observation showed that gme-2 exhibited early senescence compared with wild type, while gme-1/gme-2 displayed a much more severe early senescence phenotype (Fig. 7A). As a decreased chlorophyll content is a typical physiological marker for senescence in plants, we measured the chlorophyll contents of our gme mutants. As shown in Fig. 7B, the chlorophyll contents of 15-day-old gme-2 and gme-1/gme-2 plants were similar to that in wild type, but the levels decreased more quickly than in wild type at later stages of growth (e.g., days 20, 25 and 30).
The gme mutants exhibited early leaf senescence. (A) The leaves of 6-week-old WT, gme-2, gme-1/gme-2, gme-2 P GME ::GME and gme-1/gme-2 P GME ::GME plants. (B) Relative chlorophyll content in the fifth leaf of 15-, 20-, 25- or 30-day-old WT, gme-2, gme-1/gme-2, gme-2 P GME ::GME and gme-1/gme-2 P GME ::GME plants. Error bars represent the SE (n = 3). t-test: *P < 0.05, **P < 0.01. (C–H) Real-time PCR analysis of the expression levels of CAB1 (C), CAB2 (D), RBCS (E), SAG13 (F), SAG21 (G) and SEN4 (H). ACTIN2 was used as an internal control. Error bars represent the SE (n = 3). Asterisks represent Student’s t-test significance compared with wild type (*P < 0.05, **P < 0.01).
We next examined the expression of senescence-reduced marker genes, including CHLOROPHYLL A/B-BINDING PROTEIN 1 (CAB1), CAB2 and RUBISCO SMALL SUBUNIT (RBCS), and three senescence-induced genes, SENESCENCE-ASSOCIATED GENE 13 (SAG13), SAG21 and SENESCENCE 4 (SEN4)29,30,31,32,33, in WT and gme mutant plants. Consistent with the observed physiological phenotype, the expression of CAB1, CAB2 and RBCS was obviously down-regulated in gme-2 and gme-1/gme-2 (Fig. 7C–E), whereas the expression of SAG13, SAG21 and SEN4 was up-regulated in gme-2 and gme-1/gme-2 (Fig. 7F–H).
Further analysis showed that GME could rescue the chlorophyll level and expression of CAB1, CAB2, RBCS, SAG13, SAG21 and SEN4 in gme-2 and gme-1/gme-2 (Fig. 7). Taken together (Fig. 7), these results suggest that GME affects leaf senescence.
Discussion
GME converts GDP-D-mannose to GDP-L-Gal and GDP-L-gulose, which are intermediates of L-ascorbate biosynthesis13,14,15. GDP-L-Gal is also a precursor of the cell wall polysaccharide RGII16, 17. Previous studies showed that the knock-down of both tomato GMEs (SIGME1 and SIGME2) by RNAi increased the level of mannose, decreased the contents of the precursors Gal and L-ascorbate, reduced the amount of RGI galactan side chains and down-regulated the cross-linking of RGII and methyl esterification of pectins in stems, resulting in retarded plant growth, leaf bleaching, fragility and reduced fruit size19, 28. SIGME1 and SIGME2 control reproductive development and vegetative growth separately34. In this study, through analysis of various T-DNA insertional mutants of Arabidopsis GME/gme-1, GME/gme-2, gme-2, gme-1/gme-2, and the transgenic complementation lines gme-1 P GME ::GME, gme-2 P GME ::GME and gme-1/gme-2 P GME ::GME, we show that GME controls male gametophyte transmission, plant growth and senescence in Arabidopsis.
Although both tomato SIGME1 and Arabidopsis GME control male gametophyte development, they control different stages of male reproductive development. Firstly, SIGME1-RNAi plants exhibited reduced pollen fertility34, while pollen grains containing gme-1 from the heterozygote GME/gme-1 failed to germinate and transmit (Figs 2 and 3, and Table 1), demonstrating that Arabidopsis GME controls haploid gametophyte development. Secondly, pollen grains carrying the gme-1 mutation developed to the tricellular stage but were unable to germinate; in comparison, pollen grains carrying the gme-2 mutation could germinate, but they produced short pollen tubes (Figs 2 and 3). On the other hand, SIGME1-RNAi resulted in a reduced density of pollen grains, which usually arrested at the tetrad stage but displayed germination rates above 60%34.
Interestingly, the defects in pollen germination and pollen tube elongation were not rescued by application of L-ascorbate or GDP-L-Gal in the gme mutants (Fig. 4 and Supplemental Fig. 4), even though boric acid was able to rescue the in muro cross-linking capacity of cell wall polysaccharides and restore the growth defects of the Arabidopsis gme-1/gme-2 mutant (Fig. 6). Consistently, the absence of ascorbate (in the vtc2 vtc5 mutant) also had no effect on male gametophyte transmission (Supplemental Table 1). These findings suggest that GME regulates pollen germination and pollen tube elongation independent of both ascorbate biosynthesis and the in muro cross-linking of cell wall polysaccharides. The formation and modification of cell wall pectins, including RGI and RGII, affect pollen tube elongation35,36,37. It would be interesting to analyse the cell wall components of pollen grains from gme mutant plants in order to identify those components that are essential for GME-regulated pollen germination and pollen tube elongation.
Arabidopsis GME was expressed in diverse tissues, including roots, stems, leaves, flowers, pollen grains and pollen tubes (Fig. 1). Consistently, the gme mutants exhibited various growth defects, including reduced rosette leaf size, dwarfism, thinner stems, short siliques, reduced numbers of seeds in siliques and defects in pollen germination and pollen tube elongation (Figs 3, 5 and 6). The defects in vegetative growth of the gme mutants could be suppressed by the application of boric acid, but not L-ascorbate (Fig. 6), demonstrating that GDP-L-Gal is essential for GME-regulated plant growth, consistent with previous studies in tomato19, 28. However, the retarded growth of the vtc2 vtc5 double mutant, another ascorbate biosynthesis mutant, could be rescued by the application of L-ascorbate, but not boric acid (Supplemental Fig. 5), suggesting that L-ascorbate is also essential for plant growth. This discrepancy between gme and vtc2 vtc5 plants requires further study.
Methods
Plant materials and growth conditions
The Arabidopsis thaliana T-DNA insertion lines CS827235 (gme-1), SALK_008960 (gme-2), CS876707 (vtc2-2), SALK_135468 (vtc5-2)2 and qrt1 27 were obtained from the Arabidopsis Biological Resource Center (ABRC, Columbus, OH). The mutants GME/gme-1 qrt/qrt and vtc2-2 vtc5–2 were generated by crossing. Pollen grains from gme-2 were used to pollinate GME/gme-1 to generate the gme-1/gme-2 mutant, and one-half of the progeny of gme-1/gme-2 were gme-1/gme-2. The primers used for the verification of gme-1, gme-2, vtc2-2 and vtc5-2 are listed in Supplemental Table 2. The primer pairs gme-1 (CS827235)-LP1/SAILB3, gme-2 (SALK_008960)-RP2/SalkLBb1.3, vtc2-2 (CS876707)-RP/SAILB3 and vtc5-2 (SALK_135468)-RP/SalkLBb1.3 were used to verify the T-DNA insertions in gme-1, gme-2, vtc2-2 and vtc5-2, respectively. Arabidopsis seeds were disinfected with bleach, plated on Murashige and Skoog (MS) medium and transferred to a greenhouse under a 16-h-light (22–24 °C)/8-h-dark (17–19 °C) photoperiod after being chilled for 3 days at 4 °C. For the boric acid, AsA and L-Gal supplementation assays, Arabidopsis seeds were disinfected and plated on boric acid-free MS medium supplemented without or with ASA, L-Gal and boric acid, respectively, for about 3 weeks, then the phenotypes of the seedlings were recorded.
Pollen analysis
Pollen grains at floral stage 13 were harvested for morphological analysis by environmental scanning electron microscopy (FEI Quanta 200; FEI Co., Hillsboro, OR), stained with a DAPI solution (0.1 M sodium phosphate, pH 7, 0.4 µg/mL of DAPI, 1 mM EDTA and 0.1% Triton X-100) for the observation of pollen nuclei by fluorescence microscopy, Alexander staining solution38 or 0.5 µg/µL of fluorescein diacetate and 1 µg/µL of propidium iodide for pollen viability testing and with 0.02% neutral red for vacuole analysis.
Aniline blue staining of germinated pollen grains in pistils was performed as described previously39. Pollinated pistils were collected 16 h after pollination, fixed in a solution of ethanol:acetic acid (3:1) for 2 h, washed with distilled water three times, further softened with 8 M NaOH overnight and then washed with distilled water three times. The softened pistils were incubated with an aniline blue solution (0.1% aniline blue in 0.1 M K2HPO4-KOH buffer, pH 11) for about 3 h in the dark, and then observed with a Zeiss fluorescence microscope (LSM710; Carl Zeiss AG, Oberkochen, Germany).
In vitro pollen germination assay
In vitro pollen germination assays were performed as described previously40 with modifications. Pollen grains were collected from flowers that had been dehydrated at room temperature for about 1 h, spread on the surface of agar medium (0.01% boric acid, 5 mM CaCl2, 5 mM KCl, 1 mM MgSO4, 10% sucrose and 1.5% low-melting agarose, pH 7.5), germinated at 22–24 °C for 12 h and then observed under a light microscope with a CCD imaging system. At least 500 pollen grains of each genotype were analysed for pollen germination rate and pollen tube length. The pollen germination medium was added with the indicated concentrations of boric acid, ASA and GDP-L-Gal sodium salt to test their effects on gme mutant pollen germination.
GUS staining
The −2990 bp promoter region of GME was amplified from Arabidopsis genomic DNA and inserted into pBI121 using HindIII and XbaI to generate P GME ::GUS. The primers used to generate the construct are listed in Supplemental Table 2. The construct was transformed into Arabidopsis by the Agrobacterium-mediated floral dip method. Histochemical staining for GUS activity in the P GME ::GUS transgenic plants was performed as described previously41.
Generation of GME transgenic plants
The P GME ::GME construct was generated by replacing the GUS gene in P GME ::GUS with the coding sequence of GME. The primers used to generate the construct are listed in Supplemental Table 2. The P GME ::GME construct was introduced into GME/gme-1 and GME/gme-2 heterozygous plants by the Agrobacterium-mediated floral dip method to generate P GME ::GME homozygous transgenic plants in gme-1 and gme-2 backgrounds. Next, gme-1 P GME ::GME was crossed with gme-2 P GME ::GME to generate gme-1/gme-2 P GME ::GME.
Subcellular localisation of GME
The coding sequence of GME was cloned into pEGAD for fusion with GFP. Agrobacterium cells containing pEGAD or pEGAD-GME were incubated, harvested, resuspended in infiltration buffer (0.2 mM acetosyringone, 10 mM MES and 10 mM MgCl2), infiltrated into Nicotiana benthamiana leaves with a needleless syringe42 and incubated at 24 °C for about 50 h before observation for GFP fluorescence. The coding sequence of GME was cloned into pEZS to fuse it with GFP. Arabidopsis protoplasts were transformed with pEZS or pEZS-GME as described previously43 and observed for GFP fluorescence with a Zeiss microscope (LSM710; Carl Zeiss AG). The primers used to generate the constructs are listed in Supplemental Table 2.
Ascorbate content measurement
Leaves of 5-week-old Arabidopsis plants were homogenised in 6% TCA (approximately 0.2 g FW mL−1) and centrifuged at 12,000 x g for 5 min. The ascorbate and dehydroascorbate contents were determined by iron (III) reduction44. Total ascorbate represents the sum of the ascorbate and dehydroascorbate contents.
Chlorophyll content measurement
For chlorophyll content measurement, the fifth leaves of WT, gme-2, gme-1/gme-2, gme-2 P GME ::GME and gme-1/gme-2 P GME ::GME plants at different growth stages (15, 20, 25 and 30 days) were harvested and measured as described previously45.
qRT-PCR and RT-PCR analyses
For the analysis of GME expression in different plant tissues, roots, stems, rosette leaves, stem leaves and flowers from ~5-week-old Arabidopsis plants were harvested for RNA extraction, reverse transcription and subsequent qRT-PCR and RT-PCR analyses. For the qRT-PCR analysis of senescence-associated genes, leaves from 6-week-old Arabidopsis plants were harvested and used for real-time PCR. For the qRT-PCR analysis of GME in WT and gme mutant plants, 4-week-old plants and pollen grains from plants at floral stage 13 were collected, respectively. qRT-PCR analyses were performed with an ABI 7500 real-time PCR system as described previously46. The primers used for qRT-PCR and RT-PCR are listed in Supplemental Table 2. ACTIN2 was used as a normalisation or internal control.
Accession numbers
The Arabidopsis Genome Initiative numbers for the genes mentioned in this article are as follows: GME (AT5G28840), ACTIN2 (AT3G18780), QRT1 (AT5G55590), VTC2 (AT4G26850), VTC5 (AT5G55120), CAB1 (AT1G29930), CAB2 (AT1G29920), RBCS (At1g67090), SAG13 (AT2G29350), SAG21 (AT4G02380) and SEN4 (AT4G30270).
Data availability
All data generated or analysed during this study are included in this published article and the Supplementary Information files.
References
Smirnoff, N. & Wheeler, G. L. Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35, 291–314 (2000).
Dowdle, J., Ishikawa, T., Gatzek, S., Rolinski, S. & Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52, 673–689, doi:10.1111/j.1365-313X.2007.03266.x (2007).
Kotchoni, S. O., Larrimore, K. E., Mukherjee, M., Kempinski, C. F. & Barth, C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol 149, 803–815, doi:10.1104/pp.108.132324 (2009).
Zhang, C. et al. Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-mannose pyrophosphorylase gene in tomato plant. PLoS One 8, e61987, doi:10.1371/journal.pone.0061987 (2013).
Talla, S. et al. Ascorbic acid is a key participant during the interactions between chloroplasts and mitochondria to optimize photosynthesis and protect against photoinhibition. J Biosci 36, 163–173 (2011).
Barth, C., Moeder, W., Klessig, D. F. & Conklin, P. L. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134, 1784–1792, doi:10.1104/pp.103.032185 (2004).
Barth, C., Gouzd, Z. A., Steele, H. P. & Imperio, R. M. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot 61, 379–394, doi:10.1093/jxb/erp310 (2010).
Conklin, P. L., Saracco, S. A., Norris, S. R. & Last, R. L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154, 847–856 (2000).
Millar, A. H. et al. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol 133, 443–447, doi:10.1104/pp.103.028399 (2003).
Qin, C. et al. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc Natl Acad Sci USA 105, 18308–18313, doi:10.1073/pnas.0806168105 (2008).
Linster, C. L. & Clarke, S. G. L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci 13, 567–573, doi:10.1016/j.tplants.2008.08.005 (2008).
Wheeler, G. L., Jones, M. A. & Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369, doi:10.1038/30728 (1998).
Wolucka, B. A. et al. Partial purification and identification of GDP-mannose 3″, 5″-epimerase of Arabidopsis thaliana, a key enzyme of the plant vitamin C pathway. Proc Natl Acad Sci USA 98, 14843–14848, doi:10.1073/pnas.011578198 (2001).
Wolucka, B. A., Davey, M. W. & Boerjan, W. A high-performance liquid chromatography radio method for determination of L-ascorbic acid and guanosine 5′-diphosphate-l-galactose, key metabolites of the plant vitamin C pathway. Anal Biochem 294, 161–168, doi:10.1006/abio.2001.5165 (2001).
Wolucka, B. A. & Van Montagu, M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278, 47483–47490, doi:10.1074/jbc.M309135200 (2003).
Lukowitz, W. et al. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA 98, 2262–2267, doi:10.1073/pnas.051625798 (2001).
Reuhs, B. L. et al. l-Galactose replaces l-fucose in the pectic polysaccharide rhamnogalacturonan II synthesized by the l-fucose-deficient mur1 Arabidopsis mutant. Planta 219, 147–157, doi:10.1007/s00425-004-1205-x (2004).
Wolucka, B. A. & Van Montagu, M. The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: an opinion. Phytochemistry 68, 2602–2613, doi:10.1016/j.phytochem.2007.08.034 (2007).
Gilbert, L. et al. GDP-D-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J 60, 499–508, doi:10.1111/j.1365-313X.2009.03972.x (2009).
Watanabe, K., Suzuki, K. & Kitamura, S. Characterization of a GDP-D-mannose 3”,5”-epimerase from rice. Phytochemistry 67, 338–346, doi:10.1016/j.phytochem.2005.12.003 (2006).
Ma, L., Wang, Y., Liu, W. & Liu, Z. Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol Lett 36, 2331–2341, doi:10.1007/s10529-014-1598-y (2014).
Imai, T., Ban, Y., Terakami, S., Yamamoto, T. & Moriguchi, T. L-Ascorbate biosynthesis in peach: cloning of six L-galactose pathway-related genes and their expression during peach fruit development. Physiol Plant 136, 139–149, doi:10.1111/j.1399-3054.2009.01213.x (2009).
Ma, H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56, 393–434 (2005).
Sanders, P. et al. RB Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod 11, 297–322 (1999).
Twell, D. Male gametogenesis and germline specification in flowering plants. Sex Plant Reprod 24, 149–160 (2011).
McCormick, S. Control of male gametophyte development. Plant Cell 16(Suppl), S142–153, doi:10.1105/tpc.016659 (2004).
Preuss, D., Rhee, S. Y. & Davis, R. W. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264, 1458–1460 (1994).
Voxeur, A. et al. Silencing of the GDP-D-mannose 3,5-epimerase affects the structure and cross-linking of the pectic polysaccharide rhamnogalacturonan II and plant growth in tomato. J Biol Chem 286, 8014–8020, doi:10.1074/jbc.M110.198614 (2011).
Gan, S. S. & Amasino, R. M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270, 1986–1988, doi:10.1126/science.270.5244.1986 (1995).
Lohman, K. N., Gan, S. S., John, M. C. & Amasino, R. M. Molecular Analysis of Natural Leaf Senescence in Arabidopsis thaliana. Physiol Plantarum 92, 322–328, doi:10.1034/j.1399-3054.1994.920218.x (1994).
Miller, J. D., Arteca, R. N. & Pell, E. J. Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol 120, 1015–1024 (1999).
Park, J. H., Oh, S. A., Kim, Y. H., Woo, H. R. & Nam, H. G. Differential expression of senescence-associated mRNAs during leaf senescence induced by different senescence-inducing factors in Arabidopsis. Plant Mol Biol 37, 445–454 (1998).
Weaver, L. M., Gan, S., Quirino, B. & Amasino, R. M. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol 37, 455–469 (1998).
Mounet-Gilbert, L. et al. Two tomato GDP-D-mannose epimerase isoforms involved in ascorbate biosynthesis play specific roles in cell wall biosynthesis and development. J Exp Bot 67, 4767–4777, doi:10.1093/jxb/erw260 (2016).
Dumont, M. et al. The cell wall pectic polymer rhamnogalacturonan-II is required for proper pollen tube elongation: implications of a putative sialyltransferase-like protein. Ann Bot 114, 1177–1188, doi:10.1093/aob/mcu093 (2014).
Hoedemaekers, K. et al. BURSTING POLLEN is required to organize the pollen germination plaque and pollen tube tip in Arabidopsis thaliana. New Phytol 206, 255–267, doi:10.1111/nph.13200 (2015).
Liu, X. L. et al. Male gametophyte defective 4 encodes a rhamnogalacturonan II xylosyltransferase and is important for growth of pollen tubes and roots in Arabidopsis. Plant J 65, 647–660, doi:10.1111/j.1365-313X.2010.04452.x (2011).
Alexander, M. P. Differential staining of aborted and nonaborted pollen. Stain Technol 44, 117–122 (1969).
Ishiguro, S., Kawai-Oda, A., Ueda, J., Nishida, I. & Okada, K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. The Plant Cell 13, 2191–2209 (2001).
Boavida, L. C. & McCormick, S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 52, 570–582 (2007).
Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Embo J 6, 3901–3907 (1987).
Qi, T. et al. The jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. The Plant Cell 23, 1795–1814 (2011).
Yoo, S. D., Cho, Y. H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2, 1565–1572 (2007).
Kampfenkel, K., Van Montagu, M. & Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225, 165–167 (1995).
Qi, T. et al. Regulation of jasmonate-induced leaf senescence by antagonism between bHLH subgroup IIIe and IIId factors in Arabidopsis. The Plant Cell 27, 1634–1649, doi:10.1105/tpc.15.00110 (2015).
Song, S. et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. The Plant Cell 26, 263–279 (2014).
Acknowledgements
We thank the ABRC for providing mutants. This work was financially supported by the grants from the National Natural Science Foundation of China (31630085) and the Ministry of Science and Technology of China (2016YFA0500501).
Author information
Authors and Affiliations
Contributions
D.X., T.Q. S.S. and C.R. conceived the project; T.Q., Z.L., M.F., Y.C., H.T., D.W., H.G. and S.S., performed research; T.Q., C.R., S.S. and D.X. analysed the data; T.Q., C.R. S.S. and D.X. wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qi, T., Liu, Z., Fan, M. et al. GDP-D-mannose epimerase regulates male gametophyte development, plant growth and leaf senescence in Arabidopsis . Sci Rep 7, 10309 (2017). https://doi.org/10.1038/s41598-017-10765-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10765-5
This article is cited by
-
A mutation in CsGME encoding GDP-mannose 3,5-epimerase results in little and wrinkled leaf in cucumber
Theoretical and Applied Genetics (2024)
-
Molecular cloning and functional analysis of a Chrysanthemum vestitum GME homolog that enhances drought tolerance in transgenic tobacco
Scientific Reports (2022)
-
Whole Proteome Analysis of GA3 Response at Panicle Stage in Grape (Vitis vinifera) cv. Thompson Seedless
Journal of Plant Growth Regulation (2020)
-
iTRAQ-based quantitative proteomic analysis reveals dynamic changes during daylily flower senescence
Planta (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.