Abstract
Myo-inositol is a ubiquitous metabolite of plants. It is synthesized by a highly conserved enzyme L-myo-inositol phosphate synthase (MIPS; EC 5.5.1.4). Myo-inositol is well characterized during abiotic stress tolerance but its role during growth and development is unclear. In this study, we demonstrate that the apical hook maintenance and hypocotyl growth depend on myo-inositol. We discovered the myo-inositol role during hook formation and its maintenance via ethylene pathway in Arabidopsis by supplementation assays and qPCR. Our results suggest an essential requirement of myo-inositol for mediating the ethylene response and its interaction with brassinosteroid to regulate the skotomorphogenesis. A model is proposed outlining how MIPS regulates apical hook formation and hypocotyl growth.
Similar content being viewed by others
Introduction
The apical hook formation and maintenance is one of crucial developmental process in higher plants as it protects the shoot apical meristem (SAM) till seedling emergence out of the soil12. Apical hook formation occurs soon after germination and is maintained while seedlings makes their way through the soil and terminates upon exposure to light. The apical hook formation is orchestrated by a variety of hormones which leads to differential cell elongation in the hypocotyl1. Apical hook formation goes through three consecutive growth phases i.e., formation, maintenance and opening41. The plant hormones, auxin and ethylene interaction leads to differential growth in the formation of apical hook34. They have been involved in differential growth in the apical hook19,34. However, the mechanism by which ethylene triggers differential growth in the hypocotyl still far from understood.
Ethylene enhances apical hook curvature as observed upon application of exogenous ethylene17,19 in constitutive triple response1 (ctr1) mutant30 and the ethylene overproducer (eto) mutants55. Auxin and ethylene are involved in differential growth in apical hook, in which auxin results in cell expansion and hypostyle growth, while ethylene has an antagonistic effect34. Moreover, ethylene has a stimulatory effect on the auxin biosynthetic pathway51 which suggests another mode of interaction at the hormone level. As both auxin and ethylene are involved in the regulation of apical hook development, their activities are mutually coordinated.
Auxin gradient is one of critical factor for apical hook formation1,35. Arabidopsis mutants of auxin response such as TRANSPORT INHIBITOR RESPONSE 1 (TIR1) and AFB members15 and over-accumulating free auxin8,34, exhibit hookless phenotype. Exogenous treatment with auxin and of polar auxin transport inhibitors affects hook curvature34,49, which suggests that optimal auxin transport is critical for differential growth in the apical hook and is regulated by dedicated influx and efflux carriers like AUX/LAX family of auxin influx carriers1,56, the PIN family of auxin efflux carriers1,38 and by the B-type ATP-binding cassette transporters (ABC transporters)45.
Brassinosteroid (BR) is a steroid hormone which binds to receptor kinase BRI1 to initiate the signal transduction, e.g. inactivation of GSK3-like kinase; BIN2, dephosphorylation of BRASSINAZOLE RESISTANT 1 (BZR1) family transcription factor; accumulation of unphosphorylated BZR1 in the nucleus; and regulation of BR target genes9. BR mutants defective in BR synthesis like det2, cbb1, cpd shows no hook formation during skotomorphogenesis10,29,52 and at lower concentration BR stimulates stem growth21. Exogenous ethylene treatment results in altered auxin gradient either directly or change in BR biosynthesis19. All the three hormones, BR, ethylene and auxin affect each other and are necessary for apical hook formation.
Previous reports showed that MIPS has been involved in cell wall biogenesis36, auxin storage2, phytic acid synthesis3 and oligosaccharides synthesis28. MIPS is also required for PIN protein localization, polar auxin transport and auxin-regulated embryogenesis11,37. Its over-expression results in resistance towards cold, drought and salt stress in several plants along with immunity towards stem nematodes in transgenic sweet potato27,32,41,46,53,54,59. Programmed cell death (PCD) was observed in AtMIPS1 mutant which is light dependent and results in enhanced basal immunity16,43. Interestingly, Ma and coworkers in 2016 indicated the role of light signaling protein i.e. FHY3 and FAR1 in maintenance of optimal level of myo-inositol via directly binding to the promoter of MIPS1 and activating its expression39. Recent study of MIPS has revealed its critical role in growth and immunity via ethylene50.
Our investigation on etiolated seedlings of Arabidopsis show that apical hook and hypocotyl development also depends on the myo-inositol level. Myo-inositol induced hook maintenance and subsequent stimulation of ethylene biosynthetic genes and auxin transporters suggest that the ethylene effect might be mediated by myo-inositol. Furthermore, myo-inositol (MI) antagonizes brassinosteroid (BR) effects during hook formation which demonstrate an important step of regulation of BR-mediated growth and development. Thus, we conclude that differential MIPS levels are required for optimal hook formation, maintenance and hypocotyl growth.
Results
Myo-inositol maintains the apical hook
Our previous study with myo-inositol phosphate synthase (MIPS) suggest its role in ethylene response50. To further assess the role of myo-inositol (MI) in ethylene response, we analyzed the effect of myo-inositol on etiolated seedlings of Arabidopsis Col-0. Exogenous MI supplementation resulted in decrease in length of the hypocotyls and hook angle (Figs. 1F, 2) compared to control condition (Fig. 1A). We analysed three concentrations of MI and found significant decrease in magnitude of the hook angle from 0.5 to 1% MI (Fig. 1K; Supplementary Figure S1). We also investigated the triple response caused by ethylene by 1-aminocyclopropane-1-carboxylic acid (ACC) supplementation (Fig. 1B) and in combination of ACC and MI (Fig. 1G). We found an increase in number of seedlings showing exaggerated apical hook formation with increasing concentration of ACC (Fig. 1L; Supplementary Figure S2). However, MI and ACC combination resulted in declination. We also analysed development of the Atmips1 mutant during dark (Fig. 3C, and Supplementary Figure S10). We observed significant increase in hook angle in Atmips1 mutant etiolated seedlings as compared to WT (Fig. 3A,B,E). These results suggest that myo-inositol role in ethylene response and hook formation.
Effect of myo-inositol (MI), ethylene (ACC), AgNO3 brassinosteroid and brassinazole (BRZ) on hook formation and maintenance in etiolated seedling. (A–E) Photograph of shoot apices of 5-day-old wild-type etiolated seedlings grown, where indicated, on ½ MS, 1 μM ACC, 20 μM AgNO3, 100 nM EBL, 0.8 μM BRZ-containing media and (F–J) in combination of MI. (K) Apical hook angle of 5-day-old etiolated seedlings on three different concentration of MI. (L) Exaggerated apical hook phenotype of 5-day-old etiolated seedlings upon three concentration of ACC and with combination with MI. (M–O) Apical hook angle of 5-day-old etiolated seedlings upon three concentration of AgNO3, EBL, BRZ and combination of these with three MI concentration. Hook angle is measured using ImageJ, 1.8.0_172, https://imagej.nih.gov/ij/. Data shown is the average of two representative biological replicates having at least 20 seedlings; error bars represent SE. Statistical differences between control and each treatment were analyzed using Student’s t test with paired two-tailed distribution: ***P < 0.001 and **P < 0.01.
Hypocotyl length of 5-day-old etiolated seedlings upon three concentrations of MI and EBL. Hook angle is measured using ImageJ, 1.8.0_172, https://imagej.nih.gov/ij/. Data shown is the average of two representative biological replicates having at least 20 seedlings; error bars represent SE. Statistical differences between control and each treatment were analyzed using Student’s t test with paired two-tailed distribution: ***P < 0.001 and **P < 0.01.
(A) WT 5-day old etiolated seedling grown on ½ MS media without chemical treatment. (C) Atmips1 mutant 5-day old etiolated seedling grown on ½ MS media without chemical treatment. (B,D) Enlarged view of selected area. Red coloured circle depicts the selected area. (E) Apical hook angle of 5-day-old etiolated seedlings (WT, Atmips1 mutant). Hook angle is measured using ImageJ, 1.8.0_172, https://imagej.nih.gov/ij/. Data shown is the average of two representative biological replicates having at least 20 seedlings; error bars represent SE. Statistical differences between control and each treatment were analyzed using Student’s t test with paired two-tailed distribution: ***P < 0.001 and **P < 0.01.
Myo-inositol acts downstream of etr in hook formation
To address whether MI acts on ethylene biosynthesis or signaling pathway, we germinated the Arabidopsis Col-0 seeds on three different concentrations of AgNO3 and found no hook formation at all three concentrations (Fig. 1C; Supplementary Figure S3). Further, when seeds were grown on media containing different combinations of AgNO3 and MI, a decrease was observed in the hook angle with an increasing MI concentration (Fig. 1H,M; Supplementary Figure S3). Surprisingly, we observed hook angle as low as 15° at 3% MI along with decrease in hypocotyl length (Fig. 1H).
Myo-inositol antagonizes brassinosteroid (BR) response
Due to the established role of BR in hook formation during skotomorphogenesis, we tried to discover relationship between MI and BR and carried out combination assay. We observed peculiar phenotype in etiolated seedlings upon epibrassinolide (EBL) treatment i.e. randomized growth of hypocotyls. Maximum randomization was observed at 100 nM concentration of EBL (Fig. 1D) where MI antagonizes this effect (Fig. 1I; Supplementary Figure S4). MI supplementation resulted in reduced randomization and decrease in hook angle (Fig. 1N). Upon 1% MI supplementation, we observe the same without any effect on hypocotyl length (Fig. 1I) however, 3% MI resulted in decrease of hypocotyl length but normal hook formation (Supplementary Figure S4O). In addition, we also carried out the Brassinazole (BRZ) and MI combinatorial assay and decrease was observed in hypocotyl length and increase in hook angle along with open cotyledon with increasing concentration of BRZ (Fig. 1E; Supplementary Figure S5). When BRZ was supplemented with MI, etiolated seedlings showed decrease in hypocotyl growth and hook angle. We observed a hook angle as low as 52° hook formation at 0.8 μM BRZ with 3% MI concentration (Fig. 1J,O). Data thus suggests a direct link between the BR and MI.
Ethylene signaling inhibitor (AgNO3) enhance the brassinosteroid response
To investigate the effect of ethylene signaling inhibitor on BR response, we grew Arabidopsis seeds on media containing a combination of EBL and AgNO3. We found acute randomized growth of etiolated seedlings at different combination of EBL and AgNO3, which was increasing with increase in EBL and AgNO3 levels (Supplementary Figure S6). Randomization of etiolated seedlings grown at combination of 10 nM EBL and 10 μM AgNO3 (Supplementary Figure S6C) was more than 100 nM concertation of EBL (Supplementary Figure S4D). Short hypocotyl and exaggerated hook formation observed at 1 μM EBL was antagonized with supplementation of AgNO3. Similarly, we observed more randomization of hypocotyls at different combination of EBL and LiCl (Supplementary Figure S7). We thus conclude that absence of ethylene or myo-inositol during skotomorphogenesis exaggerates the BR effect.
Myo-inositol cannot evoke hook formation in the presence of auxin transport inhibitors
We next investigated the effect of MI in presence of IAA and auxin transporter inhibitor TIBA. We observed agravitropic growth of etiolated seedlings upon IAA treatment, however with MI supplementation results in decrease in hook angle and gravitropic growth (Supplementary Figure S8). We also checked the effect of TIBA on etiolated seedling and found no hook formation with no change in hypocotyl length (Fig. 4J; Supplementary Figure S9C–E). Upon MI supplementation, we observed no change in apical hook except a decrease in hypocotyl length with increasing MI concentration (Supplementary Figure S9). Maximum decrease in hypocotyl length was observed at 3% MI (Supplementary Figure S9O–Q).
Responses of etiolated arabidopsis seedlings to combination of AgNO3, BRZ, LiCl, TIBA. (A) 5-day old etiolated seedling grown on ½ MS media without chemical treatment. (C,D,I,J) Etiolated Arabidopsis Seedlings grown on 10 mM LiCl, 0.8 μM BRZ, 10 mM LiCl, 5 μM TIBA. (E,F) Etiolated seedling grown on media containing combination 20 μM AgNO3 with 10 mM LiCl and 0.8 μM BRZ. (K,L). Etiolated seedling grown on media containing in combination 0.8 μM BRZ with 10 mM LiCl and 5 μM TIBA. (B,G,H,M,N) Enlarged view of selected area. Red coloured circle depicts the selected area. (O) Percentage of etiolated seedlings showing open cotyledons in combination assays with AgNO3, BRZ, LiCl, TIBA.
Skotomorphogenesis is under control of brassinosteroid, auxin and ethylene via Myo-inositol
To delve deeper into the role myo-inositol during skotomorphogenesis, we performed combination assays with inhibitors of myo-inositol, ethylene, brassinosteroid and auxin. When we analysed the etiolated seedlings grown on AgNO3 (Fig. 1C), LiCl (Fig. 4C,I), BRZ (Fig. 4D) and TIBA (Fig. 4J) with grown on MS (Fig. 4A,B). We observed an increase in apical hook angle and open cotyledon phenotype. We also analysed the etiolated seedlings grown on combination of AgNO3 with LiCl and BRZ. We observed more numbers of etiolated seedling showing open cotyledon phenotype (Fig. 4E–H). The percentage of open cotyledon seedlings were significantly high in AgNO3 and BRZ (71%) followed by AgNO3 and LiCl (53%) when compared to AgNO3 (12%), BRZ (50%) and LiCl (0%) (Fig. 4E,F,O). A decrease in hypocotyl length was observed in presence of BRZ. A similar phenotype was observed with a combination of BRZ with LiCl and TIBA (Fig. 4K–N). Percentage of etiolated seedlings showing open cotyledon phenotype which were highest with supplementation of TIBA (98%) followed by LiCl (58%) as compared to BRZ (50%), LiCl (0%) and TIBA (0%) (Fig. 4O), however BRZ could not decrease the hypocotyl length in presence of LiCl (Fig. 4K).
Expression analysis of MIPS and hormone related genes in etiolated seedlings
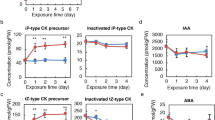
To address whether genes involved in ethylene, brassinosteroid, auxin biosynthesis, signaling or transport are regulated by myo-inositol, we carried out the quantitative RT-PCR of AtACO3 (Fig. 5B), AtCTR1 (Fig. 5C), AtERF1B (Fig. 5D), AtDET2 (Fig. 5E), AtBRI1 (Fig. 5F), AtBIN2 (Fig. 5G), AtBSL1 (Fig. 5H), AtPIN3 (Fig. 5I), AtABCB19 (Fig. 5J), and AtSAUR15 (Fig. 5K) under various concentrations of MI, EBL, ACC, AgNO3 and BRZ. We also checked the expression of myo-inositol synthesizing enzyme gene AtMIPS1 (Fig. 5A). An increase in expression of AtMIPS1 was observed upon increasing concentration of MI (Fig. 5A) indicating inducible expression of AtMIPS1 upon MI supplementation. Regulation of ethylene and brassinosteroid synthesis gene (AtACO3, AtERF1B, AtDET2) was observed upon MI and ACC treatment (Fig. 5B,D,E,H). However, BR treatment resulted in decrease in AtACO3 and AtERF1B expression till 100 nM and a distinct increase at saturating concentration of 1 μM was observed (Fig. 5B,D). As expected, feedback mechanism of gene expression in brassinosteroid and ethylene pathways was observed (Fig. 5B,D,E,F,H) except for AtBIN2. An interesting pattern was observed between AtACO3, AtERF1B and AtMIPS1 expression upon ACC treatment. We observed a bell curve in AtACO3, AtERF1B expression whereas it was an inverted bell curve in AtMIPS1 expression (Fig. 5A,B,D). Increased expression of AtPIN3 and AtABCB19 were observed only in MI and 1 μM EBL with highest in MI treated etiolated seedlings (Fig. 5I,J). We also checked the expression of early auxin-inducible gene AtSAUR15 and found its expression getting increased by the MI and EBL supplementation whereas no significant change was observed in ACC treatment (Fig. 5K). Plants expressing MIPS1 promoter fused to the uidA gene were also analysed. In 5-day-old etiolated seedlings of ProMIPS1-uidA, we observed higher β-glucuronidase (GUS) activity in MI (Fig. 6B) and BRZ (Fig. 6F) and no expression when supplemented with AgNO3 (Fig. 6D) and EBL (Fig. 6E) treated plants as compared to Control etiolated seedlings (Fig. 6A). Moreover, we observed more expression in cotyledon with no expressions in hypocotyl in ACC treated etiolated seedlings (Fig. 6C).
Relative expression of biosynthetic and signaling related genes of myo-inositol, brassinosteroid, ethylene and auxin by quantitative RT-PCR of 5-days old etiolated seedlings under different treatments. (A–L) Effect of three different concentration of MI, ACC, AgNO3, EBL and BRZ on the expression of AtMIPS1, AtACO3, AtCTR1, AtERF1B, AtDET2, AtBRI1, AtBIN2, AtBSL1, AtBAK1, AtPIN3, AtABCB19 and AtSAUR15 in 5-days old etiolated seedling grown on respective chemical agent. Data shown here is means \(\pm \)SE. Data represented are relative to GAPDH.
Discussion
Myo-inositol has numerous roles in plants i.e. cell wall biogenesis36, auxin storage2, phytic acid synthesis3, oligosaccharides synthesis28 and stress tolerance31. In this study, we demonstrate that myo-inositol is involved in hook formation during skotomorphogenesis.
Role of myo-inositol in hook formation
Ethylene is involved in hook formation and exogenous ethylene or its agonist results in exaggerated hook and shortening of hypocotyl length, as part of the classical triple response42. An increase in triple response was observed upon MI supplementation. Decrease in hook angle along with shortening of hypocotyl length was observed with increasing MI concentration. To decipher point of action of myo-inositol phosphate synthase in ethylene pathway, etiolated seedlings were supplemented with AgNO3. No hook formation was observed in etiolated seedling when treated with AgNO3 which is in accordance with the previous reports which suggests inhibition of the ethylene induced triple response7 via perturbing the ethylene perception by ETR1 and downstream signaling58. However, AgNO3 and MI combination results in hook formation in etiolated seedlings. We can therefore assume that MIPS acts downstream of ETR1 and results in hook formation. Up-regulation of ACO3 and ERF1B gene expression in etiolated seedling upon MI supplementation substantiates the hypothesis that MIPS regulates the ethylene pathway. According to previous reports, MI supplementation have no effect on MIPS expression in light grown Arabidopsis seedlings33 and it decreases MIPS expression in yeast57. In etiolated seedlings exogenous MI induces its own expression. As MIPS can be regulated by phosphorylation13, we have hypothesized that in light condition, expression of MIPS solely dependent on light however in dark its dependent on its activity which can regulated by the level of myo-inositol and hormone like Brassinosteroid.
Myo-inositol works upstream brassinosteroid response during hook formation
Involvement of brassinosteroid in hook formation directed us to investigate the effect of myo-inositol phosphate synthase during brassinosteroid signaling. We checked the response of etiolated seedling grown on media supplemented with different concentrations of EBL and found, as previously reported, randomized growth of hypocotyl along with no hook formation in etiolated seedlings22. This effect increases with increase in concentration of EBL. However, myo-inositol supplementation resulted in reduced randomization or WT phenotype. Therefore, we proposed that brassinosteroid might reduce the MI level and exogenous supplementation restores it. Upon EBL treatment, enhanced randomization of hypocotyl has been reported in auxin polar transport mutant22 and disturbing basipetal auxin transport which result in disappearance of hook18,19 suggests perturbation of polar auxin transport by higher concentration brassinosteroid. Altered polar auxin transport has also been reported in Atmips1 due to reduced level of phosphatidylinositol which affects PIN2 and PIN1 trafficking resulting in altered pattern formation11,37. Increase in expression of PIN3 and ABCB19 efflux carrier upon MI and decrease upon EBL supplementation, imply that randomized growth of etiolated seedlings upon EBL is due to disturbance of auxin transport via reducing the level of MI. Atmips1 also resembles auxin mutant37 and as GSK-3/SHAGGY like kinases are required for optimal synthesis of myo-inositol6. The presence of GSK-3/SHAGGY like kinase i.e. BIN2, a negative regulator of brassinosteroid signaling and antagonism of myo-inositol on BR effect prompted us to speculate that the BIN2 might be involved in myo-inositol synthesis via phosphorylation of serine moiety of highly conserved motif (NGSPQN)13,20,23,24,47. Inactivation of BIN2 by brassinosteroid signaling might lead to decrease in myo-inositol synthesis in dark resulting in randomized growth which is antagonized by supplementation of MI. In support of the later, we further investigated the effect of EBL in combination of LiCl on etiolated seedling and found excessive randomization even on 100 nM EBL with 10 mM of LiCl and similar result was observed combinatorial assay with EBL and AgNO3. Enhanced randomization of etiolated seedling with treatment with AgNO3 has also been reported by Gupta et al22. This study indicates that the brassinosteroid signaling decreases the ethylene response via inactivating the MIPS and results in increase in hypocotyl growth. However, treatment with saturating concentration of EBL results in antagonism probably due to a feedback mechanism5,14,40.
We checked the response of etiolated seedling in combination of BRZ and MI and found hook formation in etiolated seedlings compared to etiolated seedlings grown on only BRZ which suggests the upstream role of myo-inositol phosphate synthase to brassinosteroid in hook formation substantiating the role of MIPS in ethylene synthesis. This indicates the existence of a cross talk between ethylene, brassinosteroid and auxin at the MIPS protein level leading to myo-inositol being crucial for hook as well as proper hypocotyl development.
Myo-inositol phosphate synthase and Auxin Levels
Due to importance of differential auxin accumulation in hook formation, similar behavior of Atmips1 and Ataux1 mutant along with altered trafficking of PIN2 protein in Atmips111 led us to investigate the relationship of myo-inositol and auxin during hook formation. We carried out the combinatorial assay and found agravitropic behavior of etiolated seedlings with increase in IAA concentration. However, MI supplementation resulted in distinct decrease in hook angle and gravitropic growth. Hookless phenotype is observed upon over-accumulation of the active auxin (IAA), mutation of auxin polar transporter and treatment with inhibitor of auxin polar transport1,8. Previous report also indicates a crosstalk between ethylene and auxin in hook formation44 as inhibition of hook formation occur upon NPA treatment in eto1 and ctr1 mutant34 plus the restoration of hook occur in ethylene-insensitive mutant by auxin25. In the present study, we observe that myo-inositol was able to antagonize the effect of high level of IAA at 1 μM and 10 μM concentration. We thus conclude that myo-inositol phosphate synthase is involved in maintaining the optimal levels of auxin in the hook region via proper localization of PIN protein and ethylene synthesis which results in differential accumulation of auxin. TIBA is an auxin transport inhibitor which perturbs the auxin efflux, therefore plants treated with TIBA show agravitropic phenotype38. As expected hookless phenotype was observed when seedlings were grown in dark on media supplemented with TIBA along with agravitropic etiolated seedlings. Subsequent MI supplementation could not evoke hook formation but results in shortening of hypocotyl length. This suggests that differential auxin distribution is extreme downstream of MIPS in hook formation1,4,18,19.
Skotomorphogenesis is under control of ethylene, brassinosteroid and auxin integration via myo-inositol
Hook formation and maintenance is complex interplay of ethylene, brassinosteroid and auxin. Our investigation suggests a MI as another factor involved in hook formation. Our investigation suggests MI is an important central coordinator factor involved in hook formation and hypocotyl growth. Investigation with inhibitors of ethylene, brassinosteroid and auxin on etiolated seedling in hook formation was carried out and found increase in number of seedlings showing open cotyledon in combination compared to individual composition. It could be assumed that all the three hormones along with myo-inositol are necessary for hook formation and their action are additive in nature as we could see additive effect of inhibitors in combinations. Another observation made was on hypocotyl length i.e. brassinosteroid is required for the hypocotyl growth as decrease in hypocotyl length was seen in BRZ treated etiolated seedlings in combination of AgNO3 and TIBA whereas the MI was antagonizing the effect of brassinosteroid as increase in hypocotyl length was observed in LiCl and BRZ treated etiolated seedling. Previous reports also suggest that hypocotyl growth is associated with brassinosteroid and ethylene antagonizes brassinosteroid effect in hypocotyl growth22. It is therefore MI is one of the important regulators of apical hook formation and hypocotyl growth. A model based on findings and published data have been proposed (Fig. 7). According to which, MI induces the ethylene response which result in induction of brassinosteroid signaling and auxin gradient. Moreover, differential distribution of auxin is maintained via MI through PIN protein. Activation of brassinosteroid singling results in inhibition of MI synthesis. This will lead to inhibition of this cycle and results in opening of apical hook.
A model based on findings and published data. During hook formation, MI induces the ethylene response which induces brassinosteroid response, however this results in inhibition of MI synthesis. Differential distribution of auxin is maintained via MI through PIN protein. It is therefore MI is one of the important regulators of apical hook formation, maintenance and opening.
Material and methods
Plant sample, growth and treatments
Arabidopsis thaliana (Col-0) seeds were sterilized with sodium hypochlorite for 10 min and three time washed with RO water. Seedlings were grown on half strength MS media (DUCHEFA BIOCHEMIE) without sucrose supplemented different concentration of MI, EBL, IAA, ACC, LiCl, BRZ, TIBA, AgNO3 as specified. Seeds were then cold stratified and exposed to 12 h light stimulate uniform germination. Plates were wrapped with aluminum foil and then transferred to growth chamber for 5 days at 22 ± 1 °C.
Hook angle measurement
Apical hook angle and hypocotyl length was measured using the ImageJ software48. Apical hook angle was measured by taking hypocotyl as a reference. When hook opens up, it creates a straight line. We considered it as 180° and opening of hook as an increase in hook angle. We measured the acute angle formed between the cotyledon and hypocotyl, i.e. inner edge of the apical hook. The photographs are of the 5-day old etiolated seedlings.
Mutant confirmation
Atmips1 (salk_02779) was confirmed using left primer (LP), right primer (RP) and T-DNA-specific primers (LBb1.3) listed in Supplementary Table S1. LP, RP and LP, LBb1.3 primer combination set was used to amplify wild type and mutant PCR product which corresponds to 1054 bp and 534–834 bp, respectively. The PCR was performed with annealing temperature of 55 °C. and PCR products were then visualized on 1% agarose gels. Primers used were listed in Supplementary Table S1.
RNA isolation and cDNA synthesis
RNA was isolated from the different plant tissues (control and treated sample) using RNeasy plant mini kit (Qiagen, Germany). 5-day old control and treated etiolated seedlings were ground with liquid nitrogen and further proceeded according to the kit manual. In-column DNase treatment was done to remove the genomic DNA contamination. Quality and quantity of RNA samples were done by gel electrophoresis and nanodrop. 2 μg of RNA was used to make the cDNA using High-Capacity cDNA Reverse Transcription Kit (THERMOFISHER SCIENTIFIC) and SuperScript III First-Strand Synthesis System (THERMOFISHER SCIENTIFIC) for Full length cDNA synthesis. SYBR green PCR master mix (THERMOFISHER SCIENTIFIC) was used for qPCR analysis using primers listed in Supplementary Table S1.
GUS assay
β-Glucuronidase activity in 5-days old Arabidopsis etiolated seedlings harboring MIPS1 promoters fused with Egfp:uidA gene were checked according to the protocol described by Jefferson et al.26. 5-days old Arabidopsis seedling were harvested and dipped in GUS staining buffer for 24 h at 37 °C and plant samples were then rinsed with 70% ethanol to remove chlorophyll from the stained tissue. 5-day old etiolated seedlings grown on media supplemented with MI, ACC, AgNO3, EBL, and BRZ were GUS stained and staining was observed using stereo microscope Leica M205 A (Leica, Germany).
Statistical analyses
All values reported in this work are the average of at least two to three independent biological replicates having at least 15 seedlings each. Error bars represent SE. Statistical differences between control and each treatment were analyzed using Student’s t test with paired two-tailed distribution.
References
Abbas, M., Alabadí, D. & Blázquez, M. A. Differential growth at the apical hook: All roads lead to auxin. Front. Plant Sci. 4, 1–9 (2013).
Abreu, E. F. M. & Aragão, F. J. L. Isolation and characterization of a myo-inositol-1-phosphate synthase gene from yellow passion fruit (Passiflora edulis F. Flavicarpai) expressed during seed development and environmental stress. Ann. Bot. 99, 285–292 (2007).
Ali, N. et al. RNAi mediated down regulation of myo-inositol-3-phosphate synthase to generate low phytate rice. Rice 6, 1–12 (2013).
An, F. et al. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 22, 915–927 (2012).
Arteca, R. N., Tsai, D. & Mandava, N. B. The inhibition of brassinosteroid-induced ethylene biosynthesis in etiolated mung bean hypocotyl segments by methylpropionic acid. J. Plant Physiol. 139, 52–56 (1991).
Azab, A. N., He, Q., Ju, S., Li, G. & Greenberg, M. L. Glycogen synthase kinase-3 is required for optimal de novo synthesis of inositol. Mol. Microbiol. 63, 1248–1258 (2007).
Beyer, E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 58, 268–271 (1976).
Boerjan, W. et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7, 1405–1419 (1995).
Chaiwanon, J. & Wang, Z.-Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr. Biol. 25, 1031–1042 (2015).
Chory, J., Nagpal, P. & Peto, C. A. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 3, 445–459 (1991).
Chen, H. & Xiong, L. Myo-inositol-1-phosphate synthase is required for polar auxin transport and organ development. J. Biol. Chem. 285, 24238–24247 (2010).
Darwin, C. & Darwin, F. The Power of Movement in Plants 87–94 (D. Appleton and Co, New York, 1881).
Deranieh, R. M., He, Q., Caruso, J. A. & Greenberg, M. L. Phosphorylation regulates myo-inositol-3-phosphate synthase a novel regulatory mechanism of inositol biosynthesis. J. Biol. Chem. 288, 26822–26833 (2013).
Deslauriers, S. D. & Larsen, P. B. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 3, 626–640 (2010).
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005).
Donahue, J. L. et al. The Arabidopsis thaliana myo-inositol 1-phosphate synthase1 gene is required for myo-inositol synthesis and suppression of cell death. Plant Cell 22, 888–903 (2010).
Guzmán, P. & Ecker, J. R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 2, 513–523 (1990).
Grauwe, D. & DerStraeten, V. Auxin, ethylene and brassinosteroids: Cross talk in the Arabidopsis thaliana hypocotyl. Advances in Plant Ethylene Research: Proceedings of the 7th International Symposium on the Plant Hormone Ethylene: 115–117 (2007).
Grauwe, L., De Vandenbussche, F., Tietz, O., Palme, K. & Van Der Straeten, D. Auxin, ethylene and brassinosteroids: Tripartite control of growth in the Arabidopsis hypocotyl. Plant Cell Physiol. 46, 827–836 (2005).
Gruszka, D. The brassinosteroid signaling pathway-new key players and interconnections with other signaling networks crucial for plant development and stress tolerance. Int. J. Mol. Sci. 14, 8740–8774 (2013).
Gonzalez-Garcia, M. P. et al. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development. 138(5), 849–859 (2011).
Gupta, A., Singh, M., Jones, A. M. & Laxmi, A. Hypocotyl directional growth in Arabidopsis: A complex trait. Plant Physiol. 159, 1463–1476 (2012).
Hallchers, L. M. & Sherman, W. R. The effects of lithium ion and other agents on the activity inositol-1-phosphatase from bovine brain. J. Biol. Chem. 255, 10896–10901 (1980).
Hedgepeth, C. M. et al. Activation of the Wnt signaling pathway: A molecular mechanism for lithium action. Dev. Biol. 91, 82–91 (1997).
Hoyerová, K. et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 606, 597–606 (2010).
Jefferson, R. A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant. Mol. Biol. Rep. 5, 387–405 (1987).
Joshi, R., Ramanarao, M. V. & Baisakh, N. Arabidopsis plants constitutively overexpressing a myo-inositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol. Biochem. 65, 61–66 (2013).
Karner, U. et al. Myo-inositol and sucrose concentrations affect the accumulation of raffinose family oligosaccharides in seeds. J. Exp. Bot. 55, 1981 (2004).
Kauschmann, A. et al. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J 9, 701–713 (1996).
Kieber, J. J., Rothenberg, M., Roman, G., Feldmann, K. A. & Ecker, J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427–441 (1993).
Khurana, N., Sharma, N. & Khurana, P. Overexpression of a heat stress inducible, wheat myo-inositol-1-phosphate synthase 2 (TaMIPS2) confers tolerance to various abiotic stresses in Arabidopsis thaliana. Agri Gene 6, 24–30 (2017).
Kusuda, H., Koga, W., Kusano, M., Oikawa, A. & Saito, K. Ectopic expression of myo-inositol 3-phosphate synthase induces a wide range of metabolic changes and confers salt tolerance in rice. Plant Sci. 232, 49–56 (2015).
Latrasse, D. et al. Dual function of MIPS1 as a metabolic enzyme and transcriptional regulator. Nucleic Acids Res. 41(5), 2907–2917 (2013).
Lehman, A., Black, R. & Ecker, J. R. HOOKLESS1, an ethylene response Gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85, 183–194 (1996).
Li, H., Johnson, P., Stepanova, A., Alonso, J. M. & Ecker, J. R. Convergence of signaling of differential cell growth pathways in the control in Arabidopsis. Dev. Cell 7, 193–204 (2004).
Loewus, F. A., Kelly, S. & Neufeld, E. F. Metabolism of myo-inositol in plants: Conversion to pectin, hemicellulose, d-xylose, and sugar acids. Proc. Natl. Acad. Sci. 48, 421–425 (1962).
Luo, Y. et al. D-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in Arabidopsis and is essential for auxin-regulated embryogenesis. Plant Cell 23, 1352–1372 (2011).
Luschnig, C., Gaxiola, R. A., Grisafi, P. & Fink, G. R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187 (1998).
Ma, L. et al. Arabidopsis FHY3 and FAR1 regulate light-induced myo-inositol biosynthesis and oxidative stress responses by transcriptional activation of MIPS1. Mol. Plant 9(4), 541–557 (2016).
Mathur, J. et al. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 14, 593–602 (1998).
Majee, M. et al. A novel salt-tolerant L-myo-Inositol-1-phosphate synthase from Porteresia coarctata (Roxb.) tateoka, a halophytic wild rice. molecular cloning, bacterial overexpression, characterization, and functional introgression into tobacco-conferring salt tolerance. J. Biol. Chem. 279, 28539–28552 (2004).
Mazzella, M. A., Casal, J. J., Jorge, P. & Fox, A. R. Hormonal networks involved in apical hook development in darkness and their response to light. Front. Plant Sci. 5, 1–13 (2014).
Meng, P. H. et al. Crosstalks between myo-inositol metabolism, programmed cell death and basal immunity in Arabidopsis. PLoS One 4, 1–15 (2009).
Muday, G. K., Rahman, A. & Binder, B. M. Auxin and ethylene: Collaborators or competitors?. Trends Plant Sci. 17, 181–195 (2012).
Noh, B., Murphy, A. S. & Spalding, E. P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13, 2441–2454 (2001).
Patra, B., Ray, S. & Richter, A. Enhanced salt tolerance of transgenic tobacco plants by co-expression of PcINO1 and McIMT1 is accompanied by increased level of myo-inositol and methylated inositol. Protoplasma 245, 143–152 (2010).
Sade, Y. et al. IP3 accumulation and/or inositol depletion: Two downstream lithium’s effects that may mediate its behavioral and cellular changes. Nature 6, 1–10 (2016).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schwark, A. & Schierle, J. Interaction of ethylene and auxin in the regulation of hook growth. 1. The role of auxin in different growing regions of the hypocotyl hook of Phaseolus vulgaris. J. Plant Physiol. 140, 562–570 (1992).
Sharma, N., Chaudhary, C. & Khurana, P. Wheat Myo-inositol phosphate synthase influences plant growth and stress responses via ethylene mediated signaling. Sci. Rep. 10, 10766 (2020).
Swarup, R. et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19, 2186–2196 (2007).
Szekeres, M. et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85, 171–182 (1996).
Tan, J., Wang, C., Xiang, B., Han, R. & Guo, Z. Hydrogen peroxide and nitric oxide mediated cold- and dehydration-induced myo-inositol phosphate synthase that confers multiple resistances to abiotic stresses. Plant Cell Environ. 36, 288–299 (2013).
Wang, F. B. et al. Overexpression of IbMIPS1 gene enhances salt tolerance in transgenic sweetpotato. J. Integr. Agric. 15, 271–281 (2016).
Woeste, K. E., Ye, C. & Kieber, J. J. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 119, 521–530 (1999).
Yang, Y., Hammes, U. Z., Taylor, C. G., Schachtman, D. P. & Nielsen, E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 16, 1123–1127 (2006).
Ye, C., Bandara, W. M. & Greenberg, M. L. Regulation of inositol metabolism is fine-tuned by inositol pyrophosphates in Saccharomyces cerevisiae. J. Biol. Chem. 288, 24898–24908 (2013).
Zhao, X., Qu, X., Mathews, D. E. & Schaller, G. E. Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol. 130, 1983–1991 (2002).
Zhai, H. et al. A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 14, 592–602 (2016).
Acknowledgements
This work is supported by grants from Department of Biotechnology, Government of India (Grant number—BT/PR8406/AGIII/103/879/2013). NS acknowledges the University Grant Commission, Government of India for the UGC fellowship. The infrastructure support of the department by DST-FIST and Purse programmes is also acknowledged. We thank Glenda Gillaspy and Janet Donahue, Department of Biochemistry, Virginia Tech, Blacksburg, Virginia 24061, USA, for providing us the Arabidopsis seed material.
Author information
Authors and Affiliations
Contributions
N.S. and C.C. had performed the experiments. N.S. had prepared the all the figures. N.S. and P.K. wrote the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, N., Chaudhary, C. & Khurana, P. Role of myo-inositol during skotomorphogenesis in Arabidopsis. Sci Rep 10, 17329 (2020). https://doi.org/10.1038/s41598-020-73677-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73677-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.