Abstract
During microbial electrosynthesis (MES) driven CO2 reduction, cathode plays a vital role by donating electrons to microbe. Here, we exploited the advantage of reduced graphene oxide (RGO) paper as novel cathode material to enhance electron transfer between the cathode and microbe, which in turn facilitated CO2 reduction. The acetate production rate of Sporomusa ovata-driven MES reactors was 168.5 ± 22.4 mmol m−2 d−1 with RGO paper cathodes poised at −690 mV versus standard hydrogen electrode. This rate was approximately 8 fold faster than for carbon paper electrodes of the same dimension. The current density with RGO paper cathodes of 2580 ± 540 mA m−2 was increased 7 fold compared to carbon paper cathodes. This also corresponded to a better cathodic current response on their cyclic voltammetric curves. The coulombic efficiency for the electrons conversion into acetate was 90.7 ± 9.3% with RGO paper cathodes and 83.8 ± 4.2% with carbon paper cathodes, respectively. Furthermore, more intensive cell attachment was observed on RGO paper electrodes than on carbon paper electrodes with confocal laser scanning microscopy and scanning electron microscopy. These results highlight the potential of RGO paper as a promising cathode for MES from CO2.
Similar content being viewed by others
Introduction
Thin paper made of reduced graphene oxide (RGO) has a wide range of potential applications in research fields such as materials science, life sciences, environmental engineering, and electrochemical technologies1,2,3,4,5,6. RGO paper attracts interest because of its unique combination of physicochemical properties, which includes large surface area, tough mechanical strength, good biocompatibility, low cost as well as high flexibility, thermal stability, and electrical conductivity1, 7. In recent years, RGO papers have been explored to fabricate freestanding electrodes for electrochemical sensor applications. For instance, RGO-paper-based electrodes have been used for the detection of H2O2, glucose level in blood, pathogenic bacteria, or to monitor the secretion of nitric oxide by live cells1, 8,9,10.
Carbon paper is another paper-like material employed in electrochemical devices that share several properties with RGO papers such as high electrical conductivity, large surface area, biocompatibility, and low cost11. However, carbon paper has a lower mechanical strength than RGO paper due to its brittleness7, 12. Carbon paper has been used extensively in the field of bioelectrochemistry to fabricate electrodes for the bioelectrochemical generation of electrical energy via microbial fuel cells13,14,15,16. Carbon paper electrodes have also been employed in other bioelectrochemical devices such as bioelectric sensors and microbial electrolysis cells17,18,19.
Microbial electrosynthesis (MES) is a promising bioelectrochemical application in which the greenhouse gas CO2 is reduced into multicarbon products or methane with electrons derived from the cathode of an electrochemical reactor20,21,22,23. Until now, multicarbon compounds generated from CO2 by MES include acetate, butyrate, 2-oxobutyrate, and biofuels24,25,26. MES can be powered by electricity surpluses from the power grid as well as be partially driven by the biological oxidation of wastewater at the anode24, 27,28,29,30. MES reactors can also be integrated into bioinorganic artificial photosynthesis devices aiming at reducing CO2 with solar energy more efficiently than natural photosynthesis20, 31,32,33.
To enhance electron transfer rate and productivity of MES reactors, many efforts have focused on the development of high-performance cathode materials, microbial catalysts and growth media34,35,36,37,38,39,40,41,42,43. To construct an efficient cathode, the ideal material should possess good biocompatibility, high surface area, high durability, low production cost and high electrical conductivity44. RGO paper and carbon paper have these favorable characteristics, and thus they are used here as cathodes in a MES system with the acetogen Sporomusa ovata as the microbial catalyst for the present work. Furthermore, we have investigated the biofilm formation on both RGO and carbon paper electrodes and their electrochemical behavior in detail.
Results and Discussion
Electrode Morphology
RGO paper tested in MES reactor during this study was fabricated from high-quality GO prepared via the modified Hummer’s method13. UV-vis spectrum of GO showed characteristic peaks at 230 nm due to π-π* transitions of aromatic C-C bonds and a shoulder peak at ca. 300 nm due to n-π* transitions of C=O bonds (Fig. S1). To synthesize highly-conductive RGO paper, GO paper was first assembled and then reduced with a hydrazine solution and heating process. X-ray photoelectron spectroscopy (XPS) indicated that the reduction of GO paper into RGO paper was successful (Fig. S2). The XPS survey spectrum of RGO paper showed a higher C:O ratio than that for GO paper. Furthermore, a supplementary peak corresponding to nitrogen from the hydrazine reduction was observed at ca. 402 eV. The high resolution C1s spectrum from GO paper showed three different peaks centered at 284.2, 286.4, and 288.3 eV, corresponding to C-C in aromatic rings, C-O-C (epoxy and alkoxy), and O-C=O groups, respectively. As expected, the intensity of all peaks corresponding to carbon-oxygen groups, especially the C-O-C peak, decreased significantly for RGO paper, revealing that most oxygen-containing functional groups were removed during the reduction reaction. As expected, the intensity of all peaks corresponding to carbon-oxygen groups, especially the C-O-C peak, decreased significantly for RGO paper, revealing that most oxygen-containing functional groups were removed during the reduction reaction.
Raman spectroscopic measurements of GO and RGO paper (Fig. S3) showed that the apparent G peak position shifted by 20 cm−1 after reduction. If the apparent G peak is viewed as a superposition of G and D’ modes, then the redshift after reduction corresponds to an increase in the G peak intensity and a decrease in the D’ peak intensity45. The defect-related D/G peak intensity ratio46 is therefore decreased, which together with the decrease in D’ peak intensity are signs of increased graphitization after reduction. This is in good agreement with the results obtained from XPS. Additionally, the Raman measurement showed that the carbon paper consists of graphitic carbon (Fig. S3)47.
During the fabrication process, the thickness of RGO paper was controlled at 0.36 ± 0.02 mm. This is comparable to the 0.37 mm thickness of commercially available carbon paper (AvCarb MGL370). The diameter of 4 cm was also the same for both types of cathode (Fig. 1A and B). Both carbon paper and RGO paper tested here were freestanding and are known to have high electrical conductivity7, 48. The specific surface area measured by the BET method of the RGO paper cathode was 0.29 m2 g−1, which is 5.4 fold higher than the carbon paper cathode with similar diameter and thickness.
Furthermore, contrary to carbon paper, RGO paper can be bent or rolled exhibiting a higher flexibility (Fig. S4). All these characteristics of RGO paper make it a good cathode candidate for MES and could be exploited to increase substantially the electrode surface area available in the MES reactor for electronic interactions with microbes. In recent studies, performance improvement observed for MES reactors equipped with carbon felt or carbon cloth cathodes coated with unfunctionalized or functionalized RGO has been attributed in part to higher specific surface area and electrical conductivity compared to unmodified cathodes34, 35.
Acetate electrosynthesis from CO2 and current densities
With a carbon paper cathode poised at −690 mV versus standard hydrogen electrode (SHE), the acetate production rate from CO2 of S. ovata-driven MES reactors was 20.9 ± 5.0 mmol m−2 d−1 (Fig. 2A; Table 1). When a RGO paper cathode poised at the same potential was employed, the acetate production rate was increased to 168.5 ± 22.4 mmol m−2 d−1, which is approximately an 8 fold enhancement. Furthermore, the CO2 conversion is highly selective, as there were no multicarbon compounds other than acetate produced from CO2 in noticeable quantity in this study. The current density of 2580 ± 540 mA m−2 was 7 fold higher with the RGO paper cathode compared to the carbon paper cathode (Fig. 2B; Table 1). Coulombic efficiency for the conversion of electrons into acetate was 90.7 ± 9.3% for RGO paper cathode and 83.8 ± 4.2% for carbon paper cathode, respectively (Table 1). The improved MES performance with RGO paper cathode could be attributed to the higher specific surface area and better biocompatibility of RGO papers.
Acetate concentration, electron transferred and current consumption during MES with (A) a carbon paper cathode or (B) a RGO paper cathode. (C) Current consumption with carbon paper cathode or RGO paper cathode in sterile medium. The acetate concentration in mM corresponds to the analytical concentration of acetate in the reactor measured from high-pressure liquid chromatography (HPLC). Electron transferred curves measured from potentiostat refer to the acetate concentration in mM if all the electrons transferred were converted to acetate in the system. Results shown are from a representative example of three replicate bioelectrochemical reactors.
In the absence of S. ovata, MES reactors with either carbon or RGO paper cathode did not generate any acetate from CO2 confirming that the MES processes observed in this study were biologically-driven (Fig. 2C). Furthermore, current densities were significantly lower for abiotic MES reactors compared to MES reactors colonized by S. ovata for both materials (Table 1). Interestingly, the current density of abiotic MES reactors equipped with RGO paper cathode was 434.4 ± 53.8 mA m−2, which was 13.4-fold higher than abiotic MES reactor equipped with carbon paper cathode (Table 1). This superior current density observed with the abiotic RGO paper-equipped MES reactors is probably related to the higher specific surface area and the outstanding electrical conductivity of this material.
Cell attachment to cathode surface
SEM image of RGO paper cathode from a MES system colonized by S. ovata and running for 12 days showed a dense biofilm composed of tightly packed bacterial cells (Fig. 3A). In comparison, only scarce and isolated bacterial cells can be observed on the SEM image of the carbon paper cathode coming from a S. ovata-driven MES reactor (Fig. 3B). Confocal laser scanning microscopy (CLSM) images confirmed that a more substantial S. ovata biofilm was formed on the RGO paper cathode than on the carbon paper cathode (Fig. 3C and D). The larger number of bacterial cells present at the surface of the RGO paper cathode indicated that this material is more compatible for colonization by S. ovata in MES reactors, which led to faster acetate production and higher current density.
Cyclic voltammetry
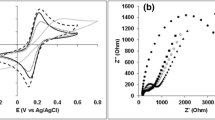
The electrochemical behavior of RGO and carbon paper cathode before and after colonization by S. ovata during MES was investigated with cyclic voltammetry (CV) (Fig. 4). There was no reversible redox peaks detected on the current–potential curves for all the tested conditions, indicating that no electroactive species were acting as electron shuttles between S. ovata and the cathodes. Furthermore, in the presence of a biofilm, the RGO paper cathode exhibited a cathodic current response at −900 mV versus Ag/AgCl ca. 8 fold and 6.7 fold higher than the colonized carbon paper cathode and the sterile control RGO paper cathode, respectively (Fig. 4). Additionally, the RGO paper cathode with sterile blank medium also exhibited a higher cathodic current response than either the carbon paper cathode with a biofilm or with sterile blank medium. These results correlated well with what has been observed with the current densities of abiotic or S. ovata-driven MES reactors equipped with either a RGO or a carbon paper cathode.
Conclusions
In conclusion, with wild type S. ovata as the microbial catalyst and at a cathode potential of −690 mV versus SHE, MES system equipped with RGO paper cathode is more performant than MES systems equipped with other types of freestanding carbonaceous cathode including carbon paper (Table 1). Besides higher acetate production rate and current density (Table 1), RGO paper cathode also has better flexibility that make it possible to be folded in the MES reactor to increase its surface area and thus improve electron exchange with microbial catalysts. Further experiments are warranted to find novel RGO paper electrode conformation that may increase the productivity of MES towards practical application.
Materials and Methods
The microbial catalyst Sporomusa ovata
S. ovata DSM 2662 wild type strain was acquired from the Deutsche Sammlung Mikroorganismen und Zellkulturen (DSMZ)49. S. ovata cultures were routinely maintained in the 311 medium with 40 mM betaine as substrate under a N2-CO2 (80:20) atmosphere. For growth with H2 as the electron source and CO2 as the carbon source, no betaine was added to the 311 medium and the atmosphere was N2-CO2-H2 (83:10:7). For all growth conditions presented in this study, casitone, sodium sulfide, yeast extract, and resazurin were omitted from the 311 medium. For MES experiments, cysteine was also omitted from the 311 medium.
Construction of freestanding reduced graphene oxide (RGO) paper cathode
High quality graphene oxide (GO) was synthesized by the modified Hummer’s method as previously reported35, 50. GO solution was then used for the fabrication of RGO paper as described before1. Briefly, 50 ml of a 1 mg/ml GO solution was sonicated for 15 minutes before being filtered with a vacuum filtration system. Then, two-dimensional GO nanosheets were assembled into a flexible layer-by-layer GO paper structure directly on the filter membrane. The thickness and layered structural orientation of the GO paper was tuned by adjusting the volume and the concentration of the GO solution added to the vacuum filtration system. Subsequently, the GO paper was reduced to obtain highly conductive RGO paper. To this end, the GO paper was put in an autoclave container along with a few drops of hydrazine solution and heated at 180 °C overnight. After cooling to room temperature, RGO paper was washed with Milli-Q water several times and annealed further at 200 °C to remove residual hydrazine as well as to enhance its electrical conductivity. After reduction and annealing, the amount of oxygen-containing groups was significantly reduced, as evidenced by the increased C/O ratio measured by X-ray photoelectron spectroscopy (XPS). The resulting RGO paper was cut into discs with a diameter of 4 cm as cathodes.
Microbial electrosynthesis of acetate and cyclic voltammetry
RGO paper and AvCarb carbon paper MGL 370 (AvCarb Material Solutions, USA) cathodes were tested at least in triplicate in three-electrode, dual chambered bioelectrochemical reactors. MES experiments were conducted at 25 °C with S. ovata DSM-2662 grown in the cathode chamber performing CO2 reduction to acetate as previously described35,36,37, 43. The RGO paper or carbon paper cathode (4 cm diameter, 0.36–0.37 mm thickness) and graphite stick anode (36 cm2) were immerged in 250 ml of 311 medium in two chambers separated by a Nafion 115 ion-exchange membrane (Ion Power, Inc., New Castle, DE, USA). The reference electrode was a sealed miniature Ag/AgCl electrode model ET072 (eDAQ, Denmark) and the cathode potential was set at −690 mV versus SHE during MES. H2-grown S. ovata cultures were established in the cathode chamber with a hydrogen-containing gas mix N2-CO2-H2 (83:10:7). The gas mix was switched to N2-CO2 (80:20) after several fresh medium swaps at which point data start being collected. During the whole MES experiment, the anode chamber was bubbled with N2-CO2. A CH Instrument potentiostat (CH Instruments, Inc, USA) was used to perform both MES as well as cyclic voltammetry (CV) experiments. During the CV experiments, the tested electrodes were scanned at a rate of 1 mV s−1 in a potential window of 0 to −1000 mV vs Ag/AgCl. Data generated during MES and CV experiments were analyzed with EC-Lab® software v.10.40 as described previously51, 52.
High-pressure liquid chromatography
Acetate concentration were measured by high-performance liquid chromatography (HPLC) with an apparatus equipped with a HPX-87H anion exchange column (Bio-Rad Laboratories Inc., California, USA) at a temperature of 30 °C. The mobile phase was 5 mM H2SO4 at a flow rate of 0.6 ml/min. Refractive index detector was used for the detection and data were analyzed with the Chromeleon software (Thermo Fisher Scientific, Denmark). Where indicated, acetate production rate was normalized with respect to the projected area of RGO paper or carbon paper cathodes.
Microscopy
For Scanning Electron Microscopy (SEM) images, cathode samples were collected and fixed during 5 hours at room temperature with a 0.1 M phosphate buffer at pH 7.0 containing 2.5% glutaraldehyde. Samples were then washed with the buffer solution without glutaraldehyde before being immerged successively in different concentration of ethanol and acetonitrile as described previously. Nitrogen-dried samples were observed with a Quanta 200 FEG scanning electron microscope (FEI) at an accelerating voltage of 10 kV under high vacuum condition. For Confocal Laser Scanning Microscopy (CLSM) images, RGO paper and carbon paper biocathodes were removed from the MES reactor and were stained with the LIVE/DEAD® BacLight™ Bacterial Viability Kit (ThermoFisher Scientific) as described previously43. CLSM image were taken with a Zeiss LSM 5 Pascal microscope and analyzed with the ZEN imaging software (Zeiss, Germany).
Analytical methods
Specific surface area of RGO paper or carbon paper cathodes was determined with the Brunauer–Emmett–Teller (BET) method as previously described53. The Agilent 8453 G1103A spectrophotometer (Agilent, Denmark) was used to measure the UV-vis spectrum of the GO solution. X-ray photoelectron spectroscopy (XPS) was performed with a Thermo Scientific™ K-Alpha™ + X-ray Photoelectron Spectrometer System with an aluminum K-Alpha (1486 eV) as x-ray source. All the samples were deposited on polished Si-wafer by drop casting for XPS measurements. X-ray spot area for measurement was set at 400 μm and flood gun was used for charge compensation. Raman spectroscopy was conducted with a Thermo Scientific DXR Raman spectrometer equipped with a 455 nm laser.
References
Zhang, M., Halder, A., Hou, C., Ulstrup, J. & Chi, Q. Free-standing and flexible graphene papers as disposable non-enzymatic electrochemical sensors. Bioelectrochemistry 109, 87–94 (2016).
Gao, H. & Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 65, 404–419 (2015).
Song, Y. et al. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 76, 195–212 (2016).
Carbone, M., Gorton, L. & Antiochia, R. An Overview of the Latest Graphene-Based Sensors for Glucose Detection: the Effects of Graphene Defects. Electroanalysis 27, 16–31 (2015).
Lawal, A. T. Synthesis and utilisation of graphene for fabrication of electrochemical sensors. Talanta 131, 424–443 (2015).
Zhang, M., Hou, C., Halder, A., Wang, H. & Chi, Q. Graphene papers: smart architecture and specific functionalization for biomimetics, electrocatalytic sensing and energy storage. Mater. Chem. Front. 1, 37–60 (2016).
Chen, H., Müller, M. B., Gilmore, K. J., Wallace, G. G. & Li, D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 20, 3557–3561 (2008).
Xiao, F., Li, Y., Gao, H., Ge, S. & Duan, H. Growth of coral-like PtAu–MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosens. Bioelectron. 41, 417–423 (2013).
Zan, X. et al. Freestanding graphene paper decorated with 2D-assembly of Au@Pt nanoparticles as flexible biosensors to monitor live cell secretion of nitric oxide. Biosens. Bioelectron. 49, 71–78 (2013).
Wang, Y., Ping, J., Ye, Z., Wu, J. & Ying, Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157:H7. Biosens. Bioelectron. 49, 492–498 (2013).
Zhou, M., Chi, M., Luo, J., He, H. & Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 196, 4427–4435 (2011).
Wei, J., Liang, P. & Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 102, 9335–9344 (2011).
Rahimnejad, M., Adhami, A., Darvari, S., Zirepour, A. & Oh, S.-E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 54, 745–756 (2015).
Jung, S. & Regan, J. M. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 77, 393–402 (2007).
Hassan, S. H. A., Kim, Y. S. & Oh, S.-E. Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzyme Microb. Technol. 51, 269–273 (2012).
Logan, B. E. & Regan, J. M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 14, 512–518 (2006).
Yuan, C.-J., Wang, C.-L., Wu, T. Y., Hwang, K.-C. & Chao, W.-C. Fabrication of a carbon fiber paper as the electrode and its application toward developing a sensitive unmediated amperometric biosensor. Biosens. Bioelectron. 26, 2858–2863 (2011).
Wang, T., Reid, R. C. & Minteer, S. D. A Paper-based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroanalysis 28, 854–859 (2016).
Liu, H., Grot, S. & Logan, B. E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 39, 4317–4320 (2005).
Lovley, D. R. & Nevin, K. P. Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 24, 385–390 (2013).
Nevin, K. P., Woodard, T. L., Franks, A. E., Summers, Z. M. & Lovley, D. R. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1, pii:e00103–10 (2010).
Rabaey, K. & Rozendal, R. A. Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 8, 706–716 (2010).
Tremblay, P.-L., Angenent, L. T. & Zhang, T. Extracellular Electron Uptake: Among Autotrophs and Mediated by Surfaces. Trends Biotechnol. 35, 360–371 (2017).
Tremblay, P.-L. & Zhang, T. Electrifying microbes for the production of chemicals. Front. Microbiol. 6, 201 (2015).
Ganigué, R., Puig, S., Batlle-Vilanova, P., Balaguer, M. D. & Colprim, J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem. Commun. 51, 3235–3238 (2015).
Li, H. et al. Integrated Electromicrobial Conversion of CO2 to Higher Alcohols. Science 335, 1596–1596 (2012).
Gong, Y. et al. Sulfide-driven microbial electrosynthesis. Environ. Sci. Technol. 47, 568–573 (2013).
Jiang, Y., Su, M. & Li, D. Removal of sulfide and production of methane from carbon dioxide in microbial fuel cells-microbial electrolysis cell (MFCs-MEC) coupled system. Appl. Biochem. Biotechnol. 172, 2720–2731 (2014).
Zeppilli, M. et al. Effect of the anode feeding composition on the performance of a continuous-flow methane-producing microbial electrolysis cell. Environ. Sci. Pollut. Res. Int. 22, 7349–7360 (2015).
Siegert, M., Li, X.-F., Yates, M. D. & Logan, B. E. The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. Front. Microbiol. 5, 778 (2014).
Zhang, T. More efficient together. Science 350, 738–739 (2015).
Liu, C. et al. Nanowire–Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals. Nano Lett. 15, 3634–3639 (2015).
Nichols, E. M. et al. Hybrid bioinorganic approach to solar-to-chemical conversion. Proc. Natl. Acad. Sci. 112, 11461–11466 (2015).
Chen, L., Tremblay, P.-L., Mohanty, S., Xu, K. & Zhang, T. Electrosynthesis of acetate from CO2 by a highly structured biofilm assembled with reduced graphene oxide–tetraethylene pentamine. J. Mater. Chem. A 4, 8395–8401 (2016).
Aryal, N., Halder, A., Tremblay, P.-L., Chi, Q. & Zhang, T. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis. Electrochim. Acta 217, 117–122 (2016).
Tremblay, P.-L., Höglund, D., Koza, A., Bonde, I. & Zhang, T. Adaptation of the autotrophic acetogen Sporomusa ovata to methanol accelerates the conversion of CO2 to organic products. Sci. Rep. 5, 16168 (2015).
Aryal, N., Tremblay, P.-L., Lizak, D. M. & Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 233, 184–190 (2017).
Ammam, F., Tremblay, P.-L., Lizak, D. M. & Zhang, T. Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata. Biotechnol. Biofuels 9, 163 (2016).
Jourdin, L. et al. A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis. J. Mater. Chem. A 2, 13093–13102 (2014).
Bajracharya, S. et al. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 195, 14–24 (2015).
Jourdin, L. et al. High Acetic Acid Production Rate Obtained by Microbial Electrosynthesis from Carbon Dioxide. Environ. Sci. Technol. 49, 13566–13574 (2015).
Nie, H. et al. Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys. Chem. Chem. Phys. 15, 14290–14294 (2013).
Zhang, T. et al. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 6, 217–224 (2013).
Guo, K., Prévoteau, A., Patil, S. A. & Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 33, 149–156 (2015).
King, A. A. K. et al. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 6, 19491 (2016).
Lucchese, M. M. et al. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 48, 1592–1597 (2010).
Ferrari, A. C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143, 47–57 (2007).
AvCarb MGL370. Available at: http://www.fuelcellstore.com/avcarb-mgl370. (Accessed: 15th May 2017).
Möller, B., Oßmer, R., Howard, B. H., Gottschalk, G. & Hippe, H. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch. Microbiol. 139, 388–396 (1984).
Zhu, N., Han, S., Gan, S., Ulstrup, J. & Chi, Q. Graphene Paper Doped with Chemically Compatible Prussian Blue Nanoparticles as Nanohybrid Electrocatalyst. Adv. Funct. Mater. 23, 5297–5306 (2013).
Lepage, G., Perrier, G., Merlin, G., Aryal, N. & Dominguez-Benetton, X. Multifactorial evaluation of the electrochemical response of a microbial fuel cell. RSC Adv. 4, 23815–23825 (2014).
Sharma, M. et al. Bioelectrocatalyzed reduction of acetic and butyric acids via direct electron transfer using a mixed culture of sulfate-reducers drives electrosynthesis of alcohols and acetone. Chem. Commun. 49, 6495–6497 (2013).
Poreddy, R., Engelbrekt, C. & Riisager, A. Copper oxide as efficient catalyst for oxidative dehydrogenation of alcohols with air. Catal. Sci. Technol. 5, 2467–2477 (2015).
Acknowledgements
T.Z. acknowledges financial support by the Novo Nordisk Foundation. Q.C. is grateful to the financial support from DFF-FTP (Project No. 12-127447). M.Z. acknowledges the CSC PhD scholarship (No. 201306170047).
Author information
Authors and Affiliations
Contributions
N.A., P.L.T. and T.Z. conceived the experiments. N.A. conducted the microbial electrosynthesis experiments. A.H., M.Z. and P.R.W. fabricated and characterized the rGO paper cathode. N.A., P.L.T., Q.C. and T.Z. interpreted the data and wrote the manuscript. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aryal, N., Halder, A., Zhang, M. et al. Freestanding and flexible graphene papers as bioelectrochemical cathode for selective and efficient CO2 conversion. Sci Rep 7, 9107 (2017). https://doi.org/10.1038/s41598-017-09841-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09841-7
This article is cited by
-
From Electricity to Products: Recent Updates on Microbial Electrosynthesis (MES)
Topics in Catalysis (2021)
-
Performance comparison between batch and continuous mode of operation of microbial electrosynthesis for the production of organic chemicals
Journal of Applied Electrochemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.