Abstract
Calcium-dependent protein kinases (CDPKs) are crucial calcium sensors that play important roles in the regulation of plant growth and developmental processes, as well as protective responses to environmental stress. Here, we identified 28 CDPK genes from barley and cloned 5 new, full-length CDPK genes, MLOC_58648a, MLOC_19618a, MLOC_71733a, AK249361a and MLOC_4965a, using their expressed sequence tags. Phylogenetic and gene structural analyses revealed that the CDPK could be divided into four subgroups. Significant site-specific altered constraints and a high evolutionary rate may have contributed to the functional divergences among CDPK gene subfamilies. Expression profiles of different tissues and developmental stages suggested that several CDPK genes are involved in the functional development of plants. Different expression levels under a variety of abiotic stresses also indicated that the CDPK family underwent functional divergence during long-term evolution. Furthermore, several CDPK genes responded to single treatments and individual CDPK genes responded to multiple treatments, suggesting that barley CDPKs may be involved in mediating cross-talk among different signalling pathways. Our data provide an important foundation for the functional and evolutionary analyses of this important gene family in barley.
Similar content being viewed by others
Introduction
Plants have evolved a series of survival mechanisms to adapt to various environmental challenges, including high salinity, drought, low temperatures and pathogens stress. Calcium (Ca2+), as a ubiquitous secondary messenger, plays important roles in plants responses to these environmental stimuli1, 2. Under stress conditions, several Ca2+ sensors or Ca2+ binding proteins can sense changes in the cytoplasmic Ca2+ concentration and further regulate downstream genes to improve plant resistance3. Plant Ca2+ sensors or Ca2+ binding proteins are complex protein families that are divided into four major classes, calmodulin, calmodulin-like proteins, calcineurin B-like proteins and calcium-dependent protein kinases (CDPK)3, 4. Among the four protein families, CDPKs are unique because their protein kinase and calmodulin-like domains are present in a single polypeptide, resulting in Ca2+-binding and Ca2+-stimulated kinase activities within an independent protein product3, 5.
Typical CDPK family members have four distinct domains: a variable N-terminal domain, a protein kinase domain, an autoinhibitory domain and a calmodulin-like domain3,4,5. The N-terminal domains often contain palmitoylation or myristoylation sites, which are key to subcellular localization and function6, and show the highest sequence divergence among CDPK domains5. Moreover, the N-terminal domain is variable, with different lengths and amino acid compositions, and determines the specific function of the individual CDPKs6, 7. The protein kinase domain contains a catalytic domain for the binding of ATP and is adjacent to the autoinhibitory junction domain. The calmodulin-like domain contains one to four EF-hand structures for Ca2+ binding4. CDPK can be activated because the Ca2+ binding leads to a change in the protein’s conformation, altering the autoinhibitory domain8.
CDPKs play important roles in plant responses to various abiotic and biotic stresses, signalling the transduction of hormones2, 4, 9, 10. In Arabidopsis, AtCPK4/11/3/6/21, as positive regulators, were involved in tolerance to salt and drought stresses11,12,13. CDPK genes from other plants, such as OsCDPK7/9/13 from rice10, 14, 15 and ZmCDPK4/12 from maize16, 17, also have similar functions in the responses to salt and drought stresses. In addition, Ulloa et al.18 showed that jasmonic acid (JA) affects CDPK activity in plant responses to Solanum tuberosum infection18. The expression levels of some CDPK genes were increased after treatment with various plant hormones, including gibberellin, auxin and abscisic acid16, 19. However, other CDPK genes act as negative regulators. For example, AtCDPK23 mutants show an increased tolerance to drought and salt stress, while overexpressing lines are more susceptible20. Thus, the functions of CDPK genes are complex in response to biotic or abiotic stress.
The genes encoding CDPKs form a multi-gene family and exist in different plant species. Genome-wide analyses have identified 34, 31, 26, 40, 30, 19, 27, 50, 41, 19, 31, 29 and 25 CDPK genes in Arabidopsis 4, rice9, wheat21, maize22, poplar23, grape24, cassava25, soybean26, cotton27, cucumber28, pepper29, tomato30 and canola31, respectively. Barley (Hordeum vulgare L.) was one of the first domesticated grains and is an important crop plants worldwide. It has a higher resistance to adverse environmental conditions than its close relative wheat32. Fedorowicz-Strońska et al.33 identified 27 CDPK genes from barley and analysed their expression levels under intensifying drought stress conditions33. However, 4 of the 27 barley CDPK genes, HvCDPK7, 9, 16 and 27, were expressed sequence tags (ESTs), implying that the protein structures were not complete. Here, we identified 28 HvCDPKs by a genome-wide analysis and cloned the full-length open reading frames (ORFs) of five novel HvCDPK genes using their EST sequences from barley. Phylogenetic and gene structural analyses were performed to determine their evolutionary relationships. We further analysed the functional divergence of this gene family and the expression profiles of HvCDPK genes in response to various abiotic stress conditions. Our results provide valuable information on the evolutionary history and the biological functions of the barley CDPK family.
Results
Identification of the HvCDPK genes in barley
In this study, a genome-wide analysis of the CDPK gene family was performed using barley genome sequences found in the Ensembl Plants and PGSB-PlantsDB databases. A total of 25 proteins, including the alternatively spliced forms, had a conserved protein kinase domain and four EF-hand domains. Proteins that were similar to CDPK-related protein, calcium/calmodulin-dependent protein, and calcium and calcium/calmodulin-dependent protein kinases were removed (Table 1). Further, the 25 protein sequences were used as query to search the draft genome and predicted mRNAs resulted in no further hits. Among those with alternative splice variants (AK362157, AK358395 and AK366527, AK364859), we selected the longest variant for further analysis. Among the low-confidence genes of barley, 10 CDPK proteins were finally identified (Table 1). One of these proteins, MLOC_67965 from morex_contig_53987, was removed because the CDS was obviously terminated by a premature stop codon, resulting in a protein missing the EF-hand domain at its C-terminus, indicating that this gene may be a pseudogene.
Isolation of HvCDPKs in the low-confidence genes of barley
To further determine full-length sequences of HvCDPKs in low-confidence genes of barley, RACE tests were performed using primers designed from the EST sequences of HvCDPKs, including MLOC_58647.1, MLOC_58648.1, MLOC_19618.2, AK354090, AK249361.1, MLOC_4965.2, MLOC_71733.1, MLOC_71734.1 and MLOC_7896.1 (Table 1). Two full-length CDSs of the MLOC_58647 and MLOC_58648 genes were cloned, and the gene sequences were aligned with the MAFFT 7.0 program. The two genes had the same nucleotide sequence, which was named MLOC_58648a (GenBank: KY008232; Figs S1a and S2). Further, chromosomal locational analyses showed that both MLOC_58647.1 and MLOC_58648.1 genes are located in the morex_contig_42856 on the chromosome 5 of barley (Table 1 and Fig. S3). Thus, the EST fragments of MLOC_58647 and MLOC_58648 may have been derived from the same gene. Meanwhile, similar results were found between MLOC_19618.2 from morex_contig_1585699, which was located on the chromosome 1 of barley, and AK354090 from morex_contig_53624, which has an unknown chromosomal location (Table 1). Thus, we predicted that MLOC_19618.2 and AK354090 represented the same CDPK gene, designated MLOC_19618a (GenBank: KY008233; Figs S1b and S2 and Tables 1 and 2), and previous studies may have contained an assembly error. The full-length cDNAs of MLOC_71733.1, MLOC_71734.1 and MLOC_7896.1 were cloned, and similar results were also found for these three EST sequences. Here, the novel CDPK gene was named MLOC_71733a (GenBank: KY008234; Fig. S1c and Table 2). In addition, the full-length cDNAs of AK249361 and MLOC_4965.2 were amplified from the EST sequence, and named AK249361a (GenBank: KY008235) and MLOC_4965a (GenBank: KY008236), respectively (Figs S1d,e and S2 and Table 2). The primer sequences are shown in Table S1. Finally, we identified 28 barley CDPKs, including 23 CDPKs from the Ensembl Plants and PGSB-PlantsDB databases (high-confidence genes) and 5 novel full-length CDPK genes (Fig. S1 and Table 2).
Characterization and chromosomal location analysis of HvCDPKs
The barley genome has 28 CDPK genes, all containing four EF-hand domains and coding sequences of 457–627 amino acids (Table 2). The 28 CDPK proteins, designated as HvCDPK1 to HvCDPK28, according to Fedorowicz-Strońska33 and their homology to CDPK genes in rice. Eight, HvCDPK6, 7, 10, 11, 16, 21, 25 and 28, of the 28 HvCDPK proteins do not contain a myristoylation motif CDPK; however, 24 of the 28 HvCDPK proteins (excluding HvCDPK6, 7, 10 and 14) have palmitoylation sites (Table 2). The chromosomal localizations of the CDPK genes were analysed based on the Ensembl Plants database. Chromosome 5 contained the maximum number of CDPK genes, which was eight (Fig. S3), followed by chromosome 2, which had 6 CDPK genes. Chromosomes 3 and 4 each contained 3 CDPK genes, while chromosomes 1 and 6 each had 2 genes, respectively. Chromosome 7 contained 1 CDPK gene (Fig. S3). HvCDPK6, 15, 18, 23 and 26 may be located in gap regions because their chromosomal locations could not be identified.
Phylogenetic relationships and gene structural analyses of the HvCDPKs
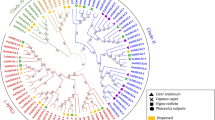
To examine the phylogenetic relationships among the HvCDPK genes and other CDPKs in plants, a neighbour-joining tree was constructed using CDPK protein sequences from barley and rice. Based on our phylogenetic results, the CDPKs were divided into four major groups (Fig. 1), consistent with previous reports23, 26, 29. The exon/intron structure of the CDPK family was analysed by comparing full-length cDNAs with their corresponding genomic sequences. The number of exons determined for members of the CDPK gene family ranged from 3 in HvCDPK6 and OsCPK6 to 12 in OsCPK4 and 18. The CDPK genes of Groups 1, 2 and 3 showed similarities in CDSs and splicing patterns, having six to seven exons, except HvCDPK6 and OsCPK6, which had three exons. HvCDPK9, 16, 21 and 24 contained four exons, as did OsCPK9. In addition, a similar exon/intron structure was found between homologous CDPK genes in barley and rice (Fig. 1). Thus, most CDPKs in the same cluster appear to have very similar exon-intron structures, strongly supporting their close evolutionary relationships and representing gene family expansion from ancient paralogs or multiple origins of gene ancestry. The CDPK genes of Group 4 had 11 to 12 exons, but we could not determine the number of exons of OsCPK31 or HvCDPK4 and 18 owing to a lack of genomic information. In addition, 20 conserved motifs within the barley CDPK genes were identified using online MEME tools (Figs 2 and S4). As mentioned above, phylogenetic analyses broadly divided the CDPK genes into four major groups. Eleven of the motifs (motif 5, 7, 10, 2, 1, 6, 12, 4, 13, 11 and 8) were shared by all of the CDPK proteins. Meanwhile, the conserved gene structures revealed unique motifs among groups (Fig. 2). For instance, motif 18 can be found in Group 1, motif 14 can be found in Group 3, and motif 19 can be found in Group 4. These results further illustrate that the function of the CDPK proteins within the same groups are similar, but there may be functional divergences between different groups.
Phylogenetic relationships among rice and barley CDPK proteins. The molecular phylogeny was constructed from a complete protein sequence alignment of CDPKs from rice and barley using the neighbour-joining method with a bootstrapping analysis (1,000 replicates). The numbers beside the branches indicate bootstrap values. The four subgroups designated from 1 to 4 are displayed in different colours.
Motif organizations in 28 HvCDPKs. The conserved motifs were detected using the MEME online tool (http://meme.sdsc.edu/meme/intro.html) and SMART (http://smart.embl-heidelberg.de/). The conserved protein kinase domain, auto-inhibitory domain and EF-hand structural domain are denoted by blue, red and green underlined, respectively.
Evolutionary history of the barley CDPK gene family
Phylogenetic analyses also showed that barley CDPKs were highly similar to their best rice matches, and 26 pairs of barley-rice CDPK proteins were putative paralogs with percentage identities ranging from 72.70% to 93.62% (Fig. 1 and Table 3). Thus, we also estimated T of 26 pairs of barley-rice putative CDPK paralogous proteins by measuring the Ks and Ka mutation rates using an r of 6.5 × 10−9 mutations per Ks site per year. The estimated Ts for barley-rice CDPK orthologs were between 30.20 to 63.20 million years ago (MYA) following the divergence of barley and rice (50–70 MYA)34. The average T of pairs of barley-rice CDPK was calculated at ~40 MYA with a standard deviation of 8 MYA. This rough dating provides an approximate time of the divergence of barley and rice CDPK genes. In addition, the Ka/Ks (ω) value was calculated for each pair of CDPK orthologous genes. The ω values for all of the putative CDPK paralogs having mean values of 0.076 were less than 1, suggesting that the 26 pairs of barley-rice CDPK proteins are under a strong purifying selection pressure (Table 3). However, three pairs of barley-rice CDPK proteins, HvCDPK9/OsCPK9 (ω = 0.1986), HvCDPK21/OsCPK21 (ω = 0.1927) and HvCDPK22/OsCPK22 (ω = 0.1813), had relatively large ω values, indicating that they may have evolved rapidly from the last common ancestor.
Analysis of functional divergence
To determine the adaptive functional diversification of the CDPK family, an analysis of type-I functional divergence between CDPK subgroups was executed using the DIVERGE 2.0 program, which evaluates the shifted evolutionary rate and altered amino acid properties. The type-I functional divergence of amino acid sites was compared between the conservative and non-conservative subgroups. As shown in Table 4, the coefficient of type-I functional divergence values varied from 0.052 to 0.352 in CDPK subgroups 1/2, 1/3, 1/4, 2/3, 2/4 and 3/4. These observations indicate that there were significant site-specific altered selective constraints on most members of the CDPK family, leading to group-specific functional evolution after diversification.
Based on the posterior probability of each comparison, the evolutionary rates at specific amino acid sites were predicted to identify sites of functional divergence among the CDPK subfamilies. To reduce false positives, a cut-off value of 0.7 was applied to identify type-I functional divergence-related residues in the CDPK subfamilies. When the CDPK sequences in the four classes were compared, 34 critical amino acid sites were predicted for Group 1/4 pairs. Thus, the functional divergence analysis suggested that, because of the differences in the numbers and distributions of predicted sites of functional divergence within each pair, the CDPK genes may be divergent from each other in their functions. In addition, we also found higher coefficient of type-I functional divergence values in CDPK subgroups 1/4 (0.352). Thus, a higher evolutionary rate may have prompted the functional divergence of CDPK genes and the evolution of new functions after divergence.
Expression profiles of barley CDPK genes at different developmental stages
Gene functions can usually be predicted by their expression profile information. The expression levels of CDPK genes were analysed using publicly available RNA-sequence data from eight different barley tissues and developmental stages32. Twenty-six of the 28 HvCDPK genes’ expression levels were obtained based on FPKM values, but FPKM data for HvCDPK10 and 18 were not found. We selected the FPKM data of the longest EST fragments of the newly acquired low-confidence HvCDPK genes, including HvCDPK16, 9, 7, 27 and 26, to analyse their expression levels (Fig. S5). A heat map was created through the hierarchical clustering of the gene expression profiles of 26 HvCDPK genes, and these could be divided into four clusters: Cluster A, B, C and D (Fig. 3). The 14 CDPK genes in Cluster A were highly expressed in eight different tissues and developmental stages, implying that Cluster A’s CDPK genes may play important roles in barley development. Cluster B’s CDPK genes were expressed most highly in the 3rd internodes of the six-leaf stage seedlings. Among Cluster C’s CDPK genes, HvCDPK25 and HvCDPK6 were almost exclusively expressed in embryos, HvCDPK21 was highly expressed in shoots and inflorescences, HvCDPK29 was highly expressed in shoots, and HvCDPK2 was highly expressed in grain, suggesting that they may be involved in the growth and development of these organs. However, four CDPK genes from Cluster D had very low expression levels throughout the sample set.
Expression profiles of barley CDPK genes in different tissues and developmental stages. Dynamic expression profiles using the FPKMs of the HvCDPK genes in different tissues and development periods. FPKM values (log2 ratio) were gene-wise normalized and hierarchically clustered using Genesis software. Genes highly or weakly expressed are colored red and green, respectively, and gray represents the FPKM value of 0. EMB; four-day-old embryos dissected from germinating grains, ROO; roots from the seedlings (10-cm shoot stage), LEA; shoots from the seedlings (10-cm shoot stage), INF1; young developing inflorescences (5 mm), INF2; developing inflorescences (1–1.5 cm), NOD; developing tillers at the six-leaf stage (3rd internode), CAR5; developing grains, with bracts removed (5 days post-anthesis), CAR15; developing grains, with bracts removed (15 days post-anthesis).
Expression profiles of HvCDPK genes during MeJA treatment
Biotic and abiotic stresses are important restrictive factors affecting plant growth and development. The identification and study of plant resistance genes can be addressed at the molecular level using gene expression profiles to reveal the mechanisms of plant resistance. To analyse the responses of HvCDPKs to MeJA treatment, the expression levels of 28 HvCDPKs were examined by qRT-PCR. As shown in Fig. 4, the expression levels of 28 HvCDPKs showed different tendencies. Further, we divided the HvCDPKs genes into four subfamilies according to how they clustered in the phylogenetic tree to test for potential functional divergences among the four groups (Fig. 4). In Group 1, 8 of the 10 HvCDPK genes, with the exceptions of HvCDPK10 and HvCDPK11, showed lower expression levels in MeJA-treated plants than in controls, and a similar trend was found in Group 4. However, transcripts of HvCDPKs in Group 2, except HvCDPK25 and HvCDPK1, increased when plants were treated with MeJA. Meanwhile, the expression levels of all the HvCDPK genes in Group 3 were greater than those of the untreated plants after 2 h of MeJA treatment. In particular, expression levels of HvCDPK22 were upregulated by more than 70-fold (12 h) compared with their respective levels at 0 h. These results indicated that the 28 HvCDPKs had different response patterns to the same stimulus (Fig. 4). Additionally, combined with analyses of functional divergence (Table 4), this suggested that the barley HvCDPK genes in the four subgroups may have different evolutionary histories, and probably novel functional divergence and adaptation.
Expression profiles of the HvCDPK genes during MeJA treatment. qPCR analyses were performed, and expression values were calculated using the 2−ΔΔCT method. Data are mean values ± SE obtained from three replicates. Different letters within a column indicate a significant difference (P < 0.05; Tukey’s test).
Expression profiles of the HvCDPK genes under abiotic stress
In plants, many CDPKs play important roles in responses to cold, salt and drought1, 35. To further evaluate the possible functional divergence of HvCDPK genes during abiotic stress, we determined the expression pattern of HvCDPK genes in response to cold (4 °C), NaCl (250 mM) and PEG (15%) treatments. Under cold stress, 5 of 10 HvCDPK genes in Group 1 (HvCDPK27, 11, 17, 28 and 24) were upregulated at 2 h after treatment. HvCDPK 6, 10 and 13 expression levels peaked at 6 h, 6 h and 12 h after treatment, respectively. Two down-regulated genes (HvCDPK7 and 25) were also identified after treatment (Fig. 5). In Group 2, four HvCDPK genes, HvCDPK2, 14, 25 and 19, were up-regulated, whereas HvCDPK26, 15, 1 and 12 were significantly down-regulated. All of the HvCDPK genes in Group 3, except for HvCDPK3 and 16, were up-regulated by cold stress. In addition, the transcript abundance of HvCDPK4 in Group 4 was up-regulated, while HvCDPK18 was down-regulated. Under salt stress, four HvCDPK genes (HvCDPK10, 27, 17 and 28) in Group 1, one HvCDPK gene (HvCDPK12) in Group 2 and five HvCDPK genes (HvCDPK8, 16, 9, 29 and 22) in Group 3 were up-regulated, whereas the rest of the HvCDPK genes were down-regulated, including HvCDPK4 and 18 in Group 4 (Fig. 5). In response to the PEG treatment, six HvCDPK genes in Group1, four HvCDPK genes in Group 2, five HvCDPK genes in Group 3 and all of the HvCDPK genes in Group 4 were up-regulated (Fig. 5). In addition, by in situ hybridization, HvCDPK1, 16, 9 and 10 transcripts were detected in the leaves of barley under different treatments. As shown in Fig. 6a, strong signal of HvCDPK1, 16, 9 and 10 genes were found in the control samples, respectively. Compared with the control, HvCDPK9 and 10 showed stronger signal, but HvCDPK1 showed weaker signal after JA, cold, salt and drought treatments. Moreover, HvCDPK16 were up-regulated following JA treatment. These changes in signal of genes expressing tissueregions were consistent with the changes in the overall expression level demonstrated by qRT-PCR. Thus, several HvCDPK genes responded to a treatment, and individual HvCDPKs responded to multiple treatments (Fig. 6b and c), suggesting that barley CDPKs may be involved in mediating cross-talk among different signalling pathways.
Localization of CDPK transcripts and cross-talk among different signalling pathways. (a) Longitudinal section of leaves probed with DIG-labeled antisense HvCDPK1, 16, 9 and 10 transcripts. Bar = 100 μm. Venn diagram showing the overlap of HvCDPK gene up-regulation (b) and down regulation (c) expression in response to MeJA, cold, salt and PEG treatments.
Discussion
CDPKs have been found only in plants and some protozoans, and play vital roles in plant growth and development, as well as abiotic and biotic stress responses. CDPKs have been identified in the genome information of several plants, including Arabidopsis 4, rice36, wheat21, cassava25, grape24, poplar23, maize22, canola31, cucumber28, pepper29 and soybean26. The draft barley genome provided valuable new information for the identification and research on CDPK genes or/and the gene family in barley. However, determining the number of CDPK gene family members also posed a challenge because of low-confidence barley genes, which were identified as potential gene fragments. Until recently, 27 members of the CDPK gene family had been identified in barley. However, 4 of the 27 CDPK protein structures were incomplete33. In this study, we investigated members of the CDPK family based on barley whole-genome data, including Ensembl Plants and PGSB-PlantsDB databases. In total, 25 full-length CDPK genes were obtained from the barley genome, including two alternatively spliced transcripts (Tables 1 and 2). In addition, five full-length cDNA were cloned using CDPK gene fragments from the low-confidence genes of the PGSB-PlantsDB barley database (Figs S1 and S2). Finally, a total of 28 CDPK sequences from barley were predicted and/or cloned in this study (Fig. 1). All 28 CDPK proteins contain protein kinase domains and 4 EF-hand calcium-binding domains, which is less than the 31 members reported in rice36 and more than the 26 members in wheat21. Research has shown that in total, 84% of the barley genome is mobile elements or other repeat structures32. In our research, tandem duplications of CDPK genes were observed in Chromosome 5, including HvCDPK27/HvCDPK25 and HvCDPK10/HvCDPK11 (Fig. S3 and Tables 1 and 2), which may be involved in the expansion of the CDPK gene family in barley. However, whether the CDPK gene family has undergone expansion by segmental genome duplication events in barley still needs to be confirmed.
In terms of biochemical properties, most of the CDPK proteins were slightly acidic, with isoelectric points (pI) in the 5–7 range28. Meanwhile, a few CDPK proteins from angiosperms, which were mainly found within Group 4, had basic pIs of 8 or more3. Barley CDPK proteins in Groups 1 and 2 have similar pIs, ranging from 4.99 to 6.31. However, pIs of 9 and 8 were predicted in Group 3 (HvCDPK9 and 21, Table 2) and Group 4 (HvCDPK4, Table 2), respectively. The most divergent homologs within the CDPK gene family indicate that a basic pI may correlate with a specific subcellular localization or function of Group 4 CDPKs3, 28. Here, HvCDPK9 and 21 in Group 3, the pIs of 9.11 and 8.44, respectively, may contribute to their functional specificity because different residues have different pI values. However, this still needs to be better understood.
CDPK proteins with less than four EF-hands have been reported in both monocotyledon and dicotyledoneae, such as nine of 34 in Arabidopsis 4, four of 25 in canola31, one of 19 in cucumber28, four of 29 in tomato30, four of 50 in soybean26 and one of 31 in rice9, two of 19 in grape24, three of 30 in poplar23, four of 40 in maize22, respectively. Our investigation of gene structure showed that all 28 barley CDPK genes contain four EF-hands (Table 2). This result is similar to that of pepper26, as a dicotyledonous plant, all 31 CDPKs contain four EF-hands. Here, we did not get regular patterns about CDPK proteins with less than four EF-hands between monocotyledon and dicotyledoneae. In addition, 20 conserved motifs were identified within the barley CDPK proteins by analysing their structural diversification (Fig. 2). The structural divergence of the core proteins correlates with sequence divergence37. Our analyses revealed that most of the CDPK proteins include the conserved protein kinase domain, which is mainly composed of motifs 9, 5, 7, 10, 2, 1 and 6 (Figs 2 and S4), and a conserved auto-inhibitory domain (motif 12). However, the loss of motifs 3 and 15 were observed in the Group 4 (HvCDPK4 and 18; Fig. 2). Furthermore, motifs 4, 13, 11, 3, 15 and 8 form the four EF-hand structural domains in Groups 1–3, and motifs 4, 13, 11, 19 and 8 form the four EF-hand structural domains in Group 4. Meanwhile, motifs 18 and 14 appeared to be specific to Group 1 (except HvCDPK10, 17 and 16) and Group 3 (except HvCDPK21), respectively. Within each group, sequence divergences were found mainly in the N-terminal domains of CDPK proteins contained myristoylation and palmitoylation sites, which may bind CDPK proteins to membranes and/or promote protein-protein interactions6, 7, 38, 39. Research has shown that CDPKs N-terminal protein undergo first modifications by acylations corresponding to N-a-acetylation and N-myristoylation, and follow by further reversible modifications such as phosphorylation or further acylations such as palmitoylation for membrane anchoring6, 7. In addition, because N-terminus lacks a cysteine residues, AtCPK3 can only be N-myristoylated, but not palmitoylated, and this effect correlated with nonspecific membrane localization and gene function of AtCPK313. In the present study, eight of CDPKs lacked myristoylation sites and four of CDPKs lacked palmitoylation sites (Table 2). However, future biochemical studies are needed to unravel the lack of N-myristoylation and/or palmitoylation site may cause subcellular localization difference and potential functional divergence of HvCDPK protein family.
Gene divergence plays an important role in the evolution of novel functions28, 40. Changes in the amino acid sites of the conserved region may result in the functional divergence of a protein family member41. In our study, the divergences in protein sequences among different subgroups indicated that the CDPK paralogues may have a variety of physiological functions. Therefore, we investigated the Type-I functional divergence between the gene groups of the CDPK family using DIVERGE software, evaluating changes in the evolutionary rate and amino acid properties41. The functional divergence analysis showed that the coefficients of the functional divergence values were more than 0 (Table 4), indicating that site-specific altered selective constraints on most members of the CDPK family led to group-specific functional evolution after diversification. The functional divergences of CDPK genes based on gene expression information have been reported in other plants, such as grapevine42 and cucumber28. To further determine the functional divergence of the barley CDPK family, we analysed the expression levels of CDPK genes in different tissues and developmental stages under different stress treatments. In non-stressed barley plants, most of the CDPKs in clusters A and B were expressed in different tissues and developmental stages (Fig. 3). Five CDPK genes in Cluster C showed tissue-specific expression. Similarly Capsicum annuum CDPK22 was expressed constitutively in roots, and CaCDPK2, 3, 4 and 31 were expressed specifically in flowers29. Moreover, some CDPK genes, such as Glycine max CDPK28 and 50, showed very low expression levels in different tissues and developmental stages, include young leaves, roots, flowers, pod, seeds and nodules26. In the present study, Cluster D showed similar expression patterns, with low expression levels across all eight samplings (Fig. 3). The duplicated genes may have different evolutionary fates, and one of the duplicates has a divergent expression pattern43. In the gene pair HvCDPK27 and HvCDPK25, tandem duplicates on Chromosome 5 (Fig. S3), had different transcriptional levels in different tissues and developmental stages (Fig. 3). Interestingly, HvCDPK25 was down-regulated, whereas HvCDPK27 was up-regulated under cold stress (Fig. 5), and HvCDPK25 was up-regulated, whereas HvCDPK27 was down-regulated under salt stress (Fig. 5). These results indicated that the homologs of CDPKs have evolved more specified functions through gene divergence to help the plant meet a broader array of lineage-specific requirements26, 28.
CDPK genes are generally induced by different hormones and various types of stress22, 23. In the present study, barley CDPK genes from the same subgroup exhibited similar expression patterns during a MeJA treatment (Fig. 4). In addition, CDPK genes were differentially expressed after cold, salt and drought stresses (Fig. 5). Thus, cross-talk may have helped regulate the signalling network of HvCDPK genes being expressed under various types of stress. Four genes, HvCDPK10, 9, 29 and 22, which were up-regulated in response to MeJA, cold, salt and drought stresses (Fig. 6a). In addition, the results of in situ hybridization analysis also confirmed that the expression level of HvCDPK9 and 10 were higher in MeJA, cold, salt and drought-treated plants than in controls (Fig. 6a). Until now, there have been no reports on the HvCDPK9 (MLOC_71733.1) and HvCDPK10 (MLOC_55774.3) gene’s resistance to environmental stress in barley. Based on the phylogenetic tree, HvCDPK9 was clustered with rice OsCPK9, which was induced by abscisic acid, PEG, NaCl and rice blast tolerance10, 44, and HvCDPK10 was clustered with rice OsCPK10, which could enhance rice resistance against Magnaporthe grisea when overexpressed45. Genes with the same functions are often closely related46, 47, indicating that HvCDPK9 and 10 play important roles in different signal transduction pathways and in the adaptation of barley to changeable environments and stresses. In addition, the expression levels of the HvCDPK29 and 22 genes were also significantly increased after cold, salt, drought and MeJA treatments. The specific roles of HvCDPK29 and 22 in barley resistance to environmental stress need further study.
In summary, we identified 28 CDPK genes in the barley genome. Significant site-specific altered constraints and a higher evolutionary rate may have contributed to the functional divergence of HvCDPKs genes. Different expression levels of HvCDPKs genes under a variety of abiotic stresses also indicated that the barley CDPK gene family has functionally diverged during long-term evolution. Further, HvCDPK9 and 10 were up-regulated by in situ hybridization analysis in barely response to MeJA, cold, salt and drought treatment, indicating that HvCDPK9 and 10 play vital role in enhancing barley tolerance to changeable environments and stresses. Our analyses provide an important foundation for understanding the potential roles of HvCDPKs in regulating barley responses to biotic and abiotic stresses. Further research on barley CDPKs’ biological functions are needed to determine their effects on plant adaptations to adverse conditions and the underlying mechanisms.
Methods
Identification of the CDPK gene family in barley
The coding (CDS) and predicted protein sequences of barley were obtained from the Ensembl Plants (http://plants.ensembl.org/Hordeum_vulgare/Info/Index) and Plant Genome and Systems Biology (PGSB)-PlantsDB (ftp://ftpmips.helmholtzmuenchen.de/plants/barley/public_data/) databases, and included 26,159 high-confidence genes and 53,220 low-confidence genes32. The domains and functional sites of all of the protein were examined using the domain analysis programs ps_scan.pl. All of the protein sequences containing protein kinase domains (PS50011) and EF-hand calcium-binding domains (PS50222) were extracted and used to search against the GenBank non-redundant (Nr) protein database. Among the low-confidence genes of barley from the PGSB-PlantsDB database, all of the protein sequences with protein kinase domains (PS50011) or EF-hand calcium-binding domains (PS50222) were also extracted and used to search against the GenBank Nr protein database. Finally, based on the domain analysis, we removed the sequences of CDPK-related protein, calcium/calmodulin-dependent protein, and calcium and calcium/calmodulin-dependent protein kinases, and the remaining proteins were considered as barley CDPKs.
RNA isolation and full-length HvCDPK cloning
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from barley samples. RNA quality was characterized initially on an agarose gel and a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), following the manufacturer’s instructions. After the total RNA was isolated, DNA-free total RNA (5 μg) was used for first-strand cDNA synthesis using Superscript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Then, rapid amplification of cDNA ends (RACE) was conducted using a SMART RACE cDNA Amplification Kit (BD Biosciences Clontech, USA), following the manufacturer’s instructions. To obtain the full-length HvCDPK CDSs from a barley sample, primers were designed based on the sequencing results (3′ and 5′ RACE). The full-length HvCDPK CDSs were amplified in a total volume of 50 μL containing 3.0 U Taq DNA polymerase (Takara), 2 mM MgCl2, 1 × PCR buffer (Takara), 0.2 mM of dNTP (Takara), 0.8 µM of each primer and 1 µL of cDNA. The PCR program was as follows: 4 min at 94 °C, 33 cycles of denaturation for 30 s at 94 °C, annealing for 30 s at 52 °C and elongation for 120 s at 72 °C, followed by a final extension of 10 min at 72 °C. The PCR products were transformed and amplified in Escherichia coli DH5α cells. The positive transformants were selected through blue/white screening and then sequenced.
Sequence and phylogenetic analyses and divergence time estimation
The full-length barley CDPK cDNA sequences from low-confidence genes were translated using EidtSeq program of DNASTAR software. The EF-hand and protein kinase motifs were predicted by SMART (http://smart.embl-heidelberg.de/)48. The molecular weight (MW), theoretical pI and grand average of hydropathicity (GRAVY) were calculated using the ProtParam tool of ExPaSy (http://web.expasy.org/protparam/)49. Myristoylaton motifs were predicted using PlantsP (http://plantsp.genomics.purdue.edu/myrist.html). Palmitoylation and N-terminal acylation predictions were performed using CSS-Palm 3.050 and NetAcet 1.051 software, respectively. The MEME program was used to search for conserved motifs in the barley candidate CDPK protein sequences52. A total of 31 full-length rice9 and barley CDPK protein sequences were aligned using the MAFFT 7.0 program, and a phylogenetic reconstruction was performed by MEGA7 software using the neighbour-joining method53. Bootstrap values were estimated (with 1,000 replicates) to assess the relative support for each branch. Gene intron/exon structures were analysed using GSDS (http://gsds.cbi.pku.edu.cn/)54. The global alignment of CDPK gene pairs were performed by the MAFFT 7.0 program. The aligned sequences were subsequently transferred into original cDNA sequences using the PAL2NAL web server (http://www.bork.embl.de/pal2nal/). Synonymous (Ks) and nonsynonymous (Ka) substitution rates were estimated by the codeml program of PAML455. The divergence time (T) of barley and rice CDPK gene pairs were calculated using the formula T = Ks/2r, where r represents the divergence rate of 6.5 × 10−9 mutations per Ks site per year56.
Functional divergence analysis
Among the CDPK subgroups, the coefficients of type-I functional divergence were calculated to estimate the level of functional divergence and predict amino acid residues responsible for functional differences between any two clusters following the methods of Gu41 using the DINERGE 2.0 software according to the instruction manual41.
The differential expression profile of CDPK genes
Expression levels of CDPK genes were estimated using fragments per kilobase of exon model per million mapped reads (FPKM) values of eight different tissues and developmental stages. FPKM values were obtained from the barley genome explorer (http://apex.ipk-gatersleben.de/apex/f?p=284:10), and the normalization and hierarchical clustering analysis of gene expression patterns were performed based on Pearson coefficients with average linkage using the Genesis software (version 1.7.1)57.
Plant material and abiotic stress
Seeds of barley were cleaned and surface-sterilized in a solution of 2% sodium hypochlorite for 15 min, rinsed five times in sterilized water and germinated in plastic trays lined with wet paper towels for 36 h in the dark at 23 °C. The seedlings were grown in soil pots and 1/4 Hoagland’s nutrient solution under controlled conditions (28 °C day/25 °C night cycle, 200 mmol photons m−2 s−1 light intensity and 75–80% relative humidity). After two weeks of germination, seedlings were exposed to cold (4 °C), methyl jasmonate (MeJA) (100 μm) or high-salinity stress (250 mM NaCl), or treated with 15% (w/v) polyethylene glycol (PEG). Plants were harvested during the treatments at 2, 6 and 12 h, with 0 h as the control. All of the samples were kept at −80 °C until used for RNA isolation.
Analysis of quantitative real-time PCR (qRT-PCR)
qRT-PCRs of HvCDPKs were performed as previously described58. All of the primers were designed by Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome), applying the following parameters: 150–200 bp of PCR product size, Nr database, 57–63 °C primer melting temperatures (Tm), and H. vulgare subsp. vulgare (taxid:112509) for ‘Organism’. All of the PCR reactions were performed under the following conditions: 40 cycles of 5 s at 95 °C, 30 s at 60 °C, and 15 s at 72 °C. The barley ubiquitin gene was used as the control (GenBank: AAA62699). The primers are shown in Table S2.
In situ hybridization
Plants were grown and treated as described above. Leaves were harvested at 0 d (as control) and 2 d of the treatments and fixed in 50 mM sodium phosphate buffer (pH 7.4) containing 4% (w/v) paraformaldehyde, respectively. Histological examinations and RNA (CDPKs) in situ hybridization analyses were performed as described by Mira et al.59. 500–600 bp fragment from CDPKs coding region were amplified by PCR using specific primers, respectively (Table S3). Then, the PCR fragments were cloned into pGEM-T-easy vector (Promega) and linearized using Nco1 or Sal1 before being used for synthesis of DIG-UTP-labeled sense or antisense probes, respectively. The probes were synthesized following the procedure described in the DIG Application Manual and were detected with single nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) staining.
References
Ranty, B. et al. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Frontiers in Plant Science 7, 327 (2016).
Lanteri, M. L., Pagnussat, G. C. & Lamattina, L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. Journal of Experimental Botany 57, 1341–1351 (2006).
Hamel, L. P., Sheen, J. & Séguin, A. Ancient signals: comparative genomics of green plant CDPKs. Trends in Plant Science 19, 79–89 (2014).
Cheng, S. H., Willmann, M. R., Chen, H. C. & Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiology 129, 469–485 (2002).
Roberts, D. M. & Harmon, A. C. Calcium-modulated proteins: targets of intracellular calcium signals in higher plants. Annual review of plant biology 43, 375–414 (1992).
Simeunovic, A., Mair, A., Wurzinger, B. & Teige, M. Know where your clients are: subcellular localization and targets of calcium-dependent protein kinases. Journal of experimental botany, erw157 (2016).
Stael, S., Bayer, R. G., Mehlmer, N. & Teige, M. Protein N-acylation overrides differing targeting signals. FEBS letters 585, 517 (2011).
Snedden, W. A. & Fromm, H. Calmodulin as a versatile calcium signal transducer in plants. New Phytologist 151, 35–66 (2001).
Ray, S., Agarwal, P., Arora, R., Kapoor, S. & Tyagi, A. K. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp. indica). Molecular Genetics and Genomics 278, 493–505 (2007).
Wei, S. et al. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. Bmc Plant Biology 14, 22–22 (2014).
Zhu, S. Y. et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19, 3019–3036 (2007).
Franz, S. et al. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Molecular Plant 4, 83–96 (2011).
Mehlmer, N. et al. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant Journal 63, 484 (2010).
Saijo, Y., Hata, S., Kyozuka, J., Shimamoto, K. & Izui, K. Over-expression of a single Ca2+ -dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant Journal for Cell & Molecular Biology 23, 319–327 (2000).
Komatsu, S. et al. Over-expression of calcium-dependent protein kinase 13 and calreticulin interacting protein 1 confers cold tolerance on rice plants. Molecular Genetics & Genomics Mgg 277, 713–723 (2007).
Jiang, S. et al. A maize calcium-dependent protein kinase gene, ZmCPK4, positively regulated abscisic acid signaling and enhanced drought stress tolerance in transgenic. Arabidopsis. Plant Physiology & Biochemistry 71C, 112–120 (2013).
Wang, C. T. & Song, W. Calcium-dependent protein kinase gene ZmCPK12 from maize confers tolerance to drought and salt stresses in transgenic plants. Acta Physiologiae Plantarum 35, 1659–1666 (2013).
Ulloa, R. M., Raíces, M., Macintosh, G. C., Maldonado, S. & Téllez-Iñón, M. T. Jasmonic acid affects plant morphology and calcium-dependent protein kinase expression and activity in Solanum tuberosum. Physiologia Plantarum 115, 417–427 (2002).
Zhang, M., Liang, S. & Lu, Y. T. Cloning and functional characterization of NtCPK4, a new tobacco calcium-dependent protein kinase. Biochimica Et Biophysica Acta 1729, 174–185 (2005).
Ma, S. Y. & Wu, W. H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Molecular Biology 65, 511–518 (2007).
Li, A. L. et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Molecular Biology 66, 429–443 (2008).
Kong, X. et al. Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. Bmc Genomics 14, 447–450 (2013).
Zuo, R. et al. Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populus trichocarpa). Molecular biology reports 40, 2645–2662 (2013).
Zhang, K. et al. Genome-wide identification and expression analysis of the CDPK gene family in grape, Vitis spp. Bmc Plant Biology 15, 1–19 (2015).
Hu, W. et al. Genome-wide survey and expression analysis of the calcium-dependent protein kinase gene family in cassava. Molecular Genetics and Genomics 291, 241–253 (2016).
Hettenhausen, C. et al. Genome-wide identification of calcium-dependent protein kinases in soybean and analyses of their transcriptional responses to insect herbivory and drought stress. Scientific reports 6 (2016).
Liu, W. et al. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. Plos One 9, e98189 (2014).
Xu, X. et al. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber. Molecular Genetics & Genomics Mgg 290, 1403–1414 (2015).
cai, h. et al. Genome-wide identification and expression analysis of calcium-dependent protein kinase and its closely related kinase genes in Capsicum annuum. Frontiers in Plant Science 6 (2015).
Hu, Z. et al. Genome-wide identification and expression analysis of calcium-dependent protein kinase in tomato. Frontiers in Plant Science 7 (2016).
Zhang, H. et al. Identification, expression and interaction analyses of calcium-dependent protein kinase (CPK) genes in canola (Brassica napus L.). Bmc Genomics 15, 211–211 (2014).
Consortium, I. B. G. S. A physical, genetic and functional sequence assembly of the barley genome. Nature 491, 711–716 (2012).
Fedorowicz-Strońska, O., Koczyk, G., Kaczmarek, M., Krajewski, P. & Sadowski, J. Genome-wide identification, characterisation and expression profiles of calcium-dependent protein kinase genes in barley (Hordeum vulgare L.). Journal of Applied Genetics, 1–12 (2016).
Kellogg, E. A. Relationships of cereal crops and other grasses. Proceedings of the National Academy of Sciences 95, 2005–2010 (1998).
Ludwig, A. A., Romeis, T. & Jones, J. D. G. CDPK-mediated signalling pathways: specificity and cross-talk. Journal of Experimental Botany 55, 181–188 (2004).
Ye, S. et al. Expression profile of calcium-dependent protein kinase (CDPKs) genes during the whole lifespan and under phytohormone treatment conditions in rice (Oryza sativa L. ssp. indica). Plant Molecular Biology 70, 311–325 (2009).
Lesk, A. M. & Chothia, C. How different amino acid sequences determine similar protein structures: The structure and evolutionary dynamics of the globins. Journal of Molecular Biology 136, 225–270 (1980).
Johnson, D. R., Bhatnagar, R. S., Knoll, L. J. & Gordon, J. I. Genetic and biochemical studies of protein N-myristoylation. Annual Review of Biochemistry 63 (1994).
Hrabak, E. M. & Harmon, A. C. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiology 132, 666–680 (2003).
Hughes, A. L. The evolution of functionally novel proteins after gene duplication. Proceedings of the Royal Society B Biological Sciences 256, 119–124 (1994).
Gu, X. Maximum-likelihood approach for gene family evolution under functional divergence. Molecular biology and evolution 18, 453–464 (2001).
Fei Chen, M. F. et al. The Evolutionary history and diverse physiological roles of the grapevine calcium-dependent protein kinase gene family. Plos One 8, e80818–e80818 (2013).
Prince, V. E. & Pickett, F. B. Splitting pairs: the diverging fates of duplicated genes. Nature Reviews Genetics 3, 827–837 (2002).
Asano, T., Tanaka, N., Yang, G., Hayashi, N. & Komatsu, S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant & Cell Physiology 46, 356–366 (2005).
Fu, L., Yu, X. & An, C. Overexpression of constitutively active OsCPK10 increases Arabidopsis resistance against Pseudomonas syringae pv. tomato and rice resistance against Magnaporthe grisea. Plant physiology and biochemistry 73, 202–210 (2013).
Hu, R. et al. Comprehensive Analysis of NAC domain transcription factor gene family in Populus trichocarpa. Bmc Plant Biology 10, 1–23 (2010).
Afoufa-Bastien, D. et al. The Vitis vinifera sugar transporter gene family: phylogenetic overview and macroarray expression profiling. Bmc Plant Biology 10, 245 (2010).
Letunic, I., Doerks, T. & Bork, P. SMART: recent updates, new developments and status in 2015. Nucleic acids research 43, D257–D260 (2015).
Gasteiger, E. et al. Protein identification and analysis tools on the ExPASy server. (Springer, 2005).
Ren, J. et al. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Engineering Design and Selection 21, 639–644 (2008).
Kiemer, L., Bendtsen, J. D. & Blom, N. NetAcet: prediction of N-terminal acetylation sites. Bioinformatics 21, 1269–1270 (2005).
Bailey, T. L., Williams, N., Misleh, C. & Li, W. W. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Research 34, 369–373 (2006).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular biology and evolution, msw054 (2016).
Hu, B. et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics, btu817 (2014).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Molecular biology and evolution 24, 1586–1591 (2007).
Gaut, B. S., Morton, B. R., McCaig, B. C. & Clegg, M. T. Substitution rate comparisons between grasses and palms: synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proceedings of the National Academy of Sciences 93, 10274–10279 (1996).
Sturn, A., Quackenbush, J. & Trajanoski, Z. Genesis: cluster analysis of microarray data. Bioinformatics 18, 207–208 (2002).
Bennett, J., Hondred, D. & Register, J. C. III Keeping qRT-PCR rigorous and biologically relevant. Plant cell reports 34, 1 (2015).
Mira, M. M., Hill, R. D. & Stasolla, C. Phytoglobins improve hypoxic root growth by alleviating apical meristem cell death. Plant Physiology 4 (2016).
Acknowledgements
This work was supported financially by the Major Projects of National Natural Science Foundation of China (No. 31590823) and the National Natural Science Foundation of China (No. 31601999).
Author information
Authors and Affiliations
Contributions
Designed the experiments: Y.P.Y., X.D.S. Performed the experiments: Y.Q.Y., M.Q., X.Y., Q.L.W., Q.C. Analyzed the data: Y.Q.Y., X.D.S. Contributed reagents/materials/analysis tools: X.Y., Q.L.W., Q.C. Wrote the paper: Y.Q.Y.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Wang, Q., Chen, Q. et al. Genome-wide survey indicates diverse physiological roles of the barley (Hordeum vulgare L.) calcium-dependent protein kinase genes. Sci Rep 7, 5306 (2017). https://doi.org/10.1038/s41598-017-05646-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05646-w
This article is cited by
-
Potential Breeding Strategies for Improving Salt Tolerance in Crop Plants
Journal of Plant Growth Regulation (2023)
-
Comparative genomic analysis of the CPK gene family in Moso bamboo (Phyllostachys edulis) and the functions of PheCPK1 in drought stress
Protoplasma (2023)
-
Genome-wide identification of the calcium-dependent protein kinase gene family in Fragaria vesca and expression analysis under different biotic stresses
European Journal of Plant Pathology (2022)
-
Genome-wide investigation of calcium-dependent protein kinase gene family in pineapple: evolution and expression profiles during development and stress
BMC Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.