Abstract
Ascospores are the primary inoculum in the wheat scab fungus Fusarium graminearum that was recently shown to have sexual stage-specific A-to-I RNA editing. One of the genes with premature-stop-codons requiring A-to-I editing to encode full-length functional proteins is AMD1 that encodes a protein with a major facilitator superfamily (MFS) domain. Here, we characterized the functions of AMD1 and its UAG to UGG editing event. The amd1 deletion mutant was normal in growth and conidiation but defective in ascospore discharge due to the premature breakdown of its ascus wall in older perithecia, which is consistent with the specific expression of AMD1 at later stages of sexual development. Expression of the wild-type or edited allele of AMD1 but not un-editable allele rescued the defects of amd1 in ascospore discharge. Furthermore, Amd1-GFP localized to the ascus membrane and Amd1 orthologs are only present in ascocarp-forming fungi that physically discharge ascospores. Interestingly, deletion of AMD1 results in the up-regulation of a number of genes related to transporter activity and membrane functions. Overall, these results indicated that Amd1 may play a critical role in maintaining ascus wall integrity during ascus maturation, and A-to-I editing of its transcripts is important for ascospore discharge in F. graminearum.
Similar content being viewed by others
Introduction
Fusarium graminearum is one of the causal agents of Fusarium Head Blight (FHB) or scab, a destructive disease of wheat and barley worldwide. Besides causing severe yield losses, the pathogen often contaminates infested grains with deoxynivalenol (DON), zearalenone, and other mycotoxins1, 2. F. graminearum overwinters on plant debris and discharges ascospores into the air in the spring to infect flowering wheat or barley heads. Unlike many other plant pathogenic fungi, sexual reproduction plays a critical role in the infection cycle of F. graminearum because ascospores are the primary inoculum of FHB3, 4. Under field conditions, conidia produced on diseased plant tissues are mainly for spreading infection to vegetative tissues of host plants because of the flowering time of wheat heads.
F. graminearum is a homothallic ascomycete and a tractable genetic system for studying sexual development because of its high homologous recombination frequency and fertility5,6,7. In the past decade, numerous genes important for sexual reproduction have been identified, including a number of protein kinase, phosphatase, and transcription factor genes and other genes with diverse functions7,8,9,10. Whereas many of these genes also are important for vegetative growth and asexual reproduction, some have specific functions during sexual reproduction in F. graminearum, such GEA1 and PUK1 that have no other defects but ascospore release or morphology11, 12. Interestingly, for the two paralogs of CDK kinase Cdc2 and beta-tubulin, whereas they have overlapping function in vegetative growth, only Cdc2A and Tub1 are important for ascus and ascospore development13, 14, suggesting differences in cell cycle regulation and microtubule cytoskeleton between vegetative hyphae and ascogenous tissues in F. graminearum.
Recently, A-to-I RNA editing was found to specifically occur during sexual reproduction in F. graminearum 12. In animals, A-to-I editing catalyzed by the adenosine deaminase acting on RNA (ADAR) enzymes is the most prevalent type of RNA editing15. Although plants and fungi lack ADAR orthologs, more than 26,000 A-to-I editing sites were identified in F. graminearum, and majority of them occurred in the coding regions and caused amino acid changes12. The PUK1 protein kinase gene known to be important for ascospore development and release8 had two tandem premature stop codons UAG UAG in its open reading frame (ORF) that were edited to UGG UGG during sexual reproduction to encode full-length proteins12. Additional 69 genes with premature stop codons in their ORFs that had PUK1-like editing events in perithecia were identified in F. graminearum 12, suggesting the importance of RNA editing during sexual reproduction.
FGRRES_10094 (=FGSG_10094 of the previous annotation by the Broad Institute) was one of the five hypothetical genes with PUK1-like editing events that were selected for preliminary analysis for their roles in sexual reproduction12. In this study, we further characterized the functions of FGRRES_10094 (named AMD1 for ascus maturation and ascospore discharge 1) and its UAG to UGG editing event in ascospore development and release. The amd1 deletion mutant was defective in ascospore discharge, likely due to the premature breakdown of its ascus wall. In addition to stage-specific editing, AMD1 was specifically expressed at late stages of sexual development and its orthologs are only present in ascocarp-forming fungi. Expression of different mutant alleles of AMD1 confirmed the importance of RNA editing. Furthermore, Amd1-GFP localized to the ascus membrane and deletion of AMD1 results in the up-regulation of a number of genes related to transporter activity and membrane functions. Overall, our results indicated that AMD1 may play a critical role in maintaining ascus wall integrity and A-to-I editing of its transcripts is important for ascospore discharge and auto-inhibition of ascospore germination in F. graminearum.
Results
AMD1 encodes a protein unique to ascocarp-forming ascomycetes
The ORF of FGRRES_10094 (named AMD1 for ascus maturation and ascospore discharge 1) was predicted to contain one intron towards its 5’-end. However, our RNA-seq data12 showed that this intron was incorrectly predicted but the stop codon UAG (631–633) within it was changed to UGG by RNA editing in 97.6% of the AMD1 transcripts in perithecia harvested at 8 days post-fertilization (dpf) (Fig. 1A). The actual AMD1 ORF encodes a 1386 amino acid protein that contains a well-conserved major facilitator superfamily (MFS) domain and 11 transmembrane helixes (TM) (Fig. 1B). Interestingly, Amd1 appears to be a protein unique to ascocarp-forming ascomycetes because it lacks a distinct ortholog in the budding and fission yeasts and other Taphrinomycotina and Saccharomycotina species (Fig. 1C; Fig. S1). Amd1 orthologs are well conserved in Sordariomycetes, Dothideomycetes, and Leotiomycetes but not in Eurotiomycetes except Chaetothyriomycetidae species (Fig. 1C; Fig. S1). The distribution of Amd1 orthologs suggests that it may be functionally related to physical discharge of ascospores from asci and ascocarps (Fig. 1C).
Editing sites and domain structures of AMD1 and its phylogenetic distribution. (A) Transcripts in RNA-seq data, the predicted gene model, and observed coding region of AMD1. The actual gene model contains one stop codon UA632G in the incorrectly predicted intron that was edited to UG632G. Reads coverage from RNA-seq data was in gray shade. Blue and red vertical lines represent the edited and unedited portions of AMD1 transcripts at each editing site, with nonsynonymous editing sites marked with asterisks. (B) The Amd1 protein contains one major facilitator superfamily (MFS) domain (aa. 618–840) and 11 transmembrane helixes (TM). (C) The distribution of Amd1 orthologs is restricted to Sordariomycetes (perithecium), Dothidiomycetes (pseudothecium or ascostrama), Leotiomycetes (apothecia), Chaotothriomycetidae (ascostroma) and Eurotiomycetidae (cleistothecia) species of Eurotiomycetes.
The expression of AMD1 is specific to late stages of sexual development
Unlike in perithecia, AMD1 transcripts were rare in RNA-seq data of hyphae and conidia12, suggesting that AMD1 was almost specifically expressed in perithecia. To verify this result, we assayed AMD1 expression in PH-1 by qRT-PCR with RNA isolated from 12 h YEPD cultures and 8 dpf perithecia. Consistent with RNA-seq data, AMD1 transcription was barely detectable in vegetative hyphae but its expression increased over 1,000 folds in perithecia (Fig. 2A).
Expression of AMD1 in late stages of sexual development. (A) The expression level of AMD1 transcripts was assayed by qRT-PCR with RNA isolated from 12 h YEPD cultures (Hyp; arbitrarily set to 1) and 8 days post-fertilization (dpf) perithecia (Peri). Mean and standard deviation were calculated with data from three independent replicates. (B) The abundance of AMD1 transcripts in different sexual stages based on RNA-seq data of mating cultures collected at 1–2 dpf and perithecia sampled at 3–8 dpf. FPKM: Fragments Per Kilobase of exon per Million fragments mapped.
In RNA-seq generated with RNA isolated from mating cultures sampled at 1 and 2 dpf and perithecia collected 3–8 dpf (accession no. PRJNA384311), AMD1 expression was barely detectable at early stages but began to increase at 5 dpf (Fig. 2B). The abundance of AMD1 transcripts kept increasing from 6, 7, and 8 dpf (Fig. 2B). In comparison with 3 dpf young perithecia, the expression level of AMD1 was up-regulated over 250 folds at 8 dpf. The timing of un-regulated expression of AMD1 correlates with the ascus and ascospore development in perithecia.
Ascospore discharge is blocked in the amd1 mutant
The AMD1 gene replacement construct was generated and transformed into the wild-type strain PH-1 in a previous study12. The amd1 mutant (Table 1) was normal in vegetative growth and conidiation. In comparison with PH-1, it had no obvious defects in virulence in infection assays with corn silks and wheat heads (Fig. S2). The amd1 mutant also was normal in response to various stresses, including treatments with 0.75% SDS, 0.05% H2O2, and 0.7 M NaCl (Fig. S3). These results indicated that, consistent with stage-specific expression during sexual reproduction, AMD1 is not important for hyphae growth, asexual reproduction, virulence, and stress response.
The amd1 mutant also was normal in perithecium development and formed abundant melanized perithecia on carrot agar cultures at 7 dpf. However, ascospore cirrhi were rarely observed in mutant perithecia (Fig. 3A) even after prolonged incubation, suggesting its defects in ascospore release. To confirm this observation, we assayed ascospore discharge as previously described16. Whereas abundant ascospores were forcibly discharged from wild-type perithecia after incubation for 16 h, under the same conditions, ascospore discharge was not observed in the amd1 mutant (Fig. 3B). Therefore, AMD1 is essential for forcible discharge of ascospores from perithecia in F. graminearum.
The amd1 mutant was defective in ascospore release and ascus wall integrity. (A) Mating cultures of the wild-type PH-1 (WT), amd1 mutant, and transformants of amd1 expressing the AMD1 WT-, AMD1 TGG-, or AMD1 TAA-GFP construct were examined 8 days post-fertilization (dpf). Arrows point to cirrhi. (B) Ascospore discharge was assayed with 7 dpf perithecia of the same set of strains. Ascospores discharged from perithecia were accumulated as whitish masses when examined after incubation for 16 h. (C) The same set of strains were examined for asci and ascospores in 8 dpf perithecia. No intact asci were observed in the amd1/AMD1 TAA transformant. Bar = 20 μm. (D) Semi-thin sections of representative perithecia of PH-1 (WT) and the amd1 mutant that were fixed and stained with 0.5% (wt/vol) toluidine blue. Arrows mark the germinated ascospores. Bar = 20 μm.
AMD1 is required for ascus wall integrity
Although the amd1 mutant was defective in ascospore discharge, they formed abundant ascospores inside perithecia. However, when 8 dpf perithecia were examined, only scattered ascospores but not intact asci were observed in the amd1 mutant (Fig. 3C), suggesting the breakdown of ascus wall. Under the same conditions, fascicles of asci were present in wild-type perithecia (Fig. 3C). To verify this observation, we examined perithecia with semi-thin sections of 8 dpf perithecia. In the wild type, asci with ascospores were observed (Fig. 3D). However, only scattered ascospores but not asci were observed in mutant perithecia (Fig. 3D). Because turgor pressure inside asci is important for the forcible discharge of ascospores, the premature breakdown of ascus wall in mutant perithecia may be directly responsible for its defects in ascospore release and formation of ascospore cirrhi.
To determine the timing of ascus wall disintegration, we examined ascospores and asci in perithecia sampled at 5, 6, 7, and 8 dpf. Both the wild type and amd1 mutant strains had fascicles of asci with 8-ascospores in 5 or 6 dpf perithecia. In 7 dpf perithecia, the ascus wall begun to disintegrate and the arrangement of ascospores in asci became loose in the amd1 mutant (Fig. 4). No asci were observed in mutant perithecia at 8 dpf (Fig. 4). These results indicate that the breakdown of ascus wall began at 7 dpf and completed by 8 dpf.
Germination of amd1 ascospores inside perithecia
Similar to the wild type, the amd1 mutant still produced four-celled ascospores. However, most of the mutant ascospores had germinated inside perithecia by 12 dpf (Fig. 4). Ascospore germination also was visible in semi-thin sections of mutant perithecia sampled at 10 dpf (Fig. 3D). Under the same conditions, ascospore germination was never observed inside perithecia formed by the wild type (Fig. 4). Extensive observations with mutant perithecia showed that ascospore germination only occurred after the breakdown of the ascus wall. Germination was not observed with ascospores that were still inside intact asci. These results showed that ascus wall integrity is important for preventing ascospore germination inside perithecia in F. graminearum.
Interestingly, germ tubes were produced only from one end of mutant ascospores when they germinated inside perithecia (Fig. 5). When incubated in liquid complete medium (CM), both the wild-type and mutant ascospores first produced germ tubes from one end but germination from the other end also occurred rapidly. After incubation in CM for 6 h CM, approximately 25% of ascospores had germ tubes from both ends. The percentage of ascospores germinated from both ends increased to 85% in 10 h CM cultures (Fig. 5). These results indicated that mutant ascospores germinated in different manners under different conditions. Unlike germination in nutrient media, germination inside perithecia may involve different regulatory mechanisms.
Germination of amd1 mutant ascospores in perithecia and CM cultures. Ascospores of the wild-type strain PH-1 (WT), amd1 mutant, and amd1/AMD1 WT-GFP complemented transformant amd1/AMD1 WT were examined for germination in 12 dpf perithecia (Peri) or after incubation in complete medium (CM) at 25 °C for 6 h and 10 h. Ascospore germination was not observed in the wild type but germination from one end of ascospores was observed in the amd1 mutant. When incubated in CM, germination from both ends of ascospores increased from approximately 25% at 6 h to 85% at 10 h in both the wild type and amd1 mutant. Bar = 20 μm.
The AMD1 WT and AMD1 TGG but not AMD1 TAA alleles complement the amd1 mutant
For complementation assays, the wild-type AMD1 allele with the TA632G stop codon and its promoter region was amplified and fused with GFP to generate the AMD1 WT-GFP construct, which was then transformed into the amd1 mutant. All the resulting amd1/AMD1 WT-GFP transformants were normal in hyphal growth, conidiation, and sexual reproduction. Perithecia formed by the amd1/AMD1 WT-GFP transformants formed ascospore cirrhi and had no ascospore germination inside perithecia (Fig. 3A), indicating the complementation of amd1.
To determine the function of A632-to-I editing, we also generated the AMD1 TGG-GFP (edited) and AMD1 TAA (uneditable) constructs by introducing the A632G and G633A mutations, respectively, and transformed them into the amd1 mutant. The resulting transformants were screened by PCR and examined for defects in sexual reproduction. All the amd1/AMD1 TAA transformants had similar defects with the original amd1 mutant in ascospore release and ascus wall integrity (Fig. 3A), indicating the essential role for RNA editing in AMD1 function. However, expression of the AMD1 TGG allele fully complemented the ascospore release defects of amd1 (Fig. 3A). The amd1/AMD1 TGG-GFP transformants were normal in ascospore discharge and formed ascospore cirrhi as frequently as the wild type. Therefore, expression of AMD1 TGG, similar to the wild-type allele, fully complemented the amd1 mutant, suggesting that the unedited transcripts (2.4%) of AMD1 had no functions during sexual reproduction.
Amd1-GFP localizes to the ascus membrane
None of the amd1/AMD1 WT-GFP transformants had detectable GFP signals in vegetative hyphae and conidia (Fig. S4), which was consistent with the specific expression of AMD1 in perithecia. When perithecia of different development stages were examined, no GFP signals were observed in asci of 5 dpf perithecia or ascospores outside asci in older perithecia (Fig. 6). Amd1-GFP mainly localized to the ascus membrane in 8 dpf perithecia (Fig. 6). To our knowledge, this is the first report on proteins localizes to the ascus membrane in filamentous ascomycetes. The subcellular localization pattern of Amd1 is consistent with its TM helixes and functions in maintaining ascus wall integrity and ascospore discharge in F. graminearum.
Constitutive expression of the AMD1 TGG allele has no effects on hyphal growth and conidiation
Although AMD1 transcripts were rare in hyphae, it is possible that the existence of the UA632G stop codon is to avoid accidental expression of Amd1 proteins, which may be detrimental to vegetative growth in F. graminearum. To test this hypothesis, we generated the PRP27-AMD1 TGG-GFP construct and transformed it into the amd1 mutant. The resulting transformants had no obvious defects in vegetative growth and conidiation (Table S1). In 8 h germlings, localization of Amd1-GFP to the cytoplasm membrane was not observed but GFP signals were observed in peri-nuclear regions that may be related to the endoplasmic reticulum due to overexpression (Fig. S4). These results indicate that expression of AMD1 TGG by the strong, constitutive RP27 promoter 17, 18 had no effects on vegetative growth and asexual reproduction, and the localization of Amd1 to the ascus membrane may depend on its interacting proteins that are specifically expressed during sexual reproduction.
The expression of AMD1 is reduced in the Fgkin1 mutant
In F. graminearum, FgKin1, a microtubule affinity-regulating protein kinase (MARK), is also required for ascospore discharge and prevention of ascospore germination inside perithecia19. The Fgkin1 and amd1 mutants has similar defects in ascospore discharge and disintegration of the ascus wall (Fig. 7A). Similar to amd1, germination of ascospores from one end also was observed inside Fgkin1 perithecia19 (Fig. 7A). When assayed by qRT-PCR with RNA isolated from 7 dpf perithecia, the AMD1 expression level was reduced approximately 5 folds in the Fgkin1 mutant in comparison with that of the wild type (Fig. 7B). It is possible that the FgKin1 kinase controls ascus wall integrity by somehow regulating the expression of AMD1 in F. graminearum.
Similarity between Fgkin1 and amd1 mutants and reduced AMD1 expression in Fgkin1. (A) The Fgkin1 and amd1 mutants had similar defects in ascospore discharge, ascus wall disintegration, and ascospore germination inside perithecia. Bar = 20 μm. (B) The expression level of AMD1 was assayed by qRT-PCR with RNA isolated from 7 dpf perithecia of the wild-type PH-1 and Fgkin1 mutant. The expression level of AMD1 in PH-1 was arbitrarily set to 1. Mean and standard deviation were calculated with data from three independent replicates.
Deletion of AMD1 affects more than 300 genes expression
To identify genes affected by AMD1 deletion, we conducted RNA-seq analysis with RNA isolated from perithecia sampled at 7 dpf. In comparison with the wild type, 53 and 263 genes were up- and down-regulated over two folds, respectively, in the amd1 mutant (Table S2). Among the up-regulated genes, Gene Ontology (GO) enrichment analysis showed that 19 genes each related to transporter activity and membrane were significantly enriched (Fig. S5), suggesting that deletion of AMD1 may affect cross-membrane transportation and membrane functions. Among the down-regulated genes, approximately half of them encode hypothetical proteins or proteins of unknown functions (Table S2) and no significant enrichment of any GO terms was observed. However, several genes that may be related to cell wall synthesis, modifications, or integrity were down-regulated in the mutant, including FGRRES_12586, FGRRES_07238, FGRRES_17404, FGRRES_02262, FGRRES_10920, FGRRES_13169, and FGRRES_03674. Reduced expression of these genes may be related to the defects of amd1 in ascus wall integrity.
Discussion
The AMD1 gene requires A-to-I RNA editing during sexual reproduction to encode a full-length protein. Interestingly, its orthologs are only present in ascomycetes that form asci inside ascocarps and eject ascospores from asci. Most of the Eurotiomycetes that form cleistothecia such as Aspergillus nidulans lack AMD1 orthologs. It is tempting to speculate that AMD1 is functionally related to the physical ejection of ascospores from asci and its orthologs may evolve only in ascomycetes that are more advanced than those forming cleistothecia20. Ascospores are not ejected from naked asci formed by Taphrinomycotina and Saccharomycotina species or cleistothecia formed by Eurotiomycetes. In fact, Chaetothyriomycetidae species that have AMD1 orthologs are distinct from the rest of Eurotiomycetes by the formation of ascostroma and many of them are lichen forming fungi21.
The forcible ejection of ascospores is functionally related to the generation of turgor pressure in asci22, 23. In F. graminearum, individual mature asci extend through the ostiole prior to ascospore discharge24. It is likely that the ascus wall was degraded in the amd1 mutant before asci were mature and ready for ascospore ejection. Although its exact function is not clear, AMD1 may be involved in strengthening or the modification of ascus wall at later stages. It is also possible that mannitol accumulation and ion fluxes important for ascus turgor generation23 were affected in the amd1 mutant, which in turn may affect ascus turgor and ascus wall modifications. The specific expression of AMD1 at late sexual stages and its localization to the ascus membrane supported the likely functions of Amd1 proteins in maintaining ascus wall integrity. Furthermore, RNA-seq analysis showed that several genes related to cell wall modification or integrity were down-regulated in mutant perithecia. Interestingly, 19 genes each encoding proteins that are functionally related to transporter activity and membrane functions were upregulated in the amd1 mutant, which accounted for over two thirds of the 53 up-regulated genes. Most of these genes with up-regulated expression in amd1 had no or little expression in 8 dpf perithecia in the wild type12, suggesting that their up-regulation may be related to the breakdown of ascus wall and membrane in the mutant.
Another defect of the amd1 mutant was the germination of ascospores from one end inside perithecia after the breakdown of ascus wall. This defect is similar to that of the Fgkin1 mutant19. Kin1 is a MARK kinase that is involved in microtubule based transportation via phosphorylation of microtubule-associated proteins25. In this study, we showed that the expression level of AMD1 was decreased approximately 5 folds in the Fgkin1 mutant, which may be directly related to the defects of Fgkin1 in ascospore discharge and germination. Because Amd1 localized to the ascus membrane but FgKin1 localizes to the septal pore19, they may not directly interact with each other and AMD1 expression may be indirectly regulated by FgKIN1. Nevertheless, unlike AMD1, FgKIN1 is constitutively expressed, and the Fgkin1 mutant had a reduced growth rate19. Therefore, the FgKin1 kinase must have other downstream targets and more diverse functions than Amd1 in F. graminearum. The gea1 mutant is another mutant in F. graminearum that had similar defects with amd1 in ascospore discharge and germinati11. However, different from Amd1, Gea1 protein localizes to the cytoplasm membrane of ascospores and some gea1 ascospores had morphology defects. Nevertheless, it will be important to determine the relationships among Amd1, Fgkin1, and Gea1 during ascus maturation and ascospore ejection.
Like the Fgkin1 mutant19, ascospores of the amd1 mutant germinated from one end inside perithecia but germinated from both ends when cultured in CM. These observations suggest that the two ends of mature ascospores are not equal and the presence of nutrients may promote the production of germ tubes from both ends of ascospores in F. graminearum. However, it is puzzling how the fungus distinguishes one end from the other in four-celled ascospores derived from two rounds of mitosis and cytokinesis. It is also not clear what molecular mechanisms are responsible for the auto-inhibition of ascospore germination inside perithecia. If F. graminearum produces certain metabolites or ascospore surface compounds that function as auto-inhibitory factors to prevent ascospores from germination inside perithecia, the amd1 and Fgkin1 mutants may be defective in the production or accumulation of these compounds. It will be important to assay for defects of the amd1 and Fgkin1 mutants in the accumulation of mannitol and ions enriched inside asci4, 26, 27 if they are responsible for auto-inhibition of ascospore germination in F. graminearum.
AMD1 is one of the 60 genes with premature stop codons in the coding regions that require A-to-I editing to encode full-length proteins in F. graminearum 12. Because RNA editing is incomplete, even though the editing level was 97.6% at A632, the unedited transcripts were still present and might encode a small peptide. Nevertheless, the amd1/AMD1 TGG-GFP transformants were similar to the wild type and complemented transformant in ascospore discharge, indicating that the unedited transcripts had no detectable functions if they indeed encoded a small peptide in F. graminearum. However, there are 9 other nonsynonymous editing events identified in the AMD1 transcripts, including two in the MFS domain (Fig. 1A). Five of editing sites have editing levels higher than 90% and another 7 had editing levels ranging from 30–90%. Therefore, RNA editing not only enables AMD1 to encode a full-length functional protein but also introduces amino acid sequence variations in F. graminearum. It will be interesting to determine the functions of these nonsynonymous editing sites in AMD1.
Methods
Strains and culture conditions
The F. graminearum wild-type strain PH-128 and all the transformants generated in this study were routinely maintained on potato dextrose agar (PDA) plates at 25 °C. Conidiation in liquid carboxymethyl cellulose (CMC) medium and growth rate on complete medium (CM) plates were measured as described29, 30. To assay for defects in stress responses, final concentrations of 0.75% sodium dodecyl sulfate (SDS), 0.1% H2O2, and 0.7 M NaCl were added to CM as described8. For sexual reproduction, aerial hyphae of 5-day-old carrot agar cultures were pressed down with sterile 0.1% Tween 206, 31. Perithecia, cirrhi, asci, and ascospore discharge were examined as described16, 19. Protoplast preparation and polyethylene glycol (PEG)-mediated transformation were performed as described29. Hygromycin B (CalBiochem, La Jolla, CA, USA) and geneticin (Sigma-Aldrich, St. Louis, MO, USA) were added to the final concentration at 300 and 400 μg/ml, respectively, for transformant selection.
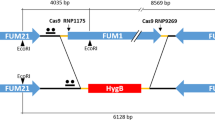
Generation of the AMD1 WT, AMD1 TGG, AMD1 TAA, and PRp27-AMD1 TGG transformants
For complementation assays, the entire AMD1 gene including its promoter region was amplified with primers 094-NF and 094-R (Table S3) and co-transformed with XhoI-digested pFL2 (carrying the geneticin resistance marker) into yeast strain XK1–25 by the gap repair approach17, 32. The AMD1 WT-GFP fusion construct was rescued from Trp+ yeast transformants and confirmed by sequencing analysis. The same yeast gap repair approach was used to generate the AMD1 TGG-GFP, PRP27 -AMD1 TGG-GFP, and AMD1 TAA-GFP constructs. To introduce the A632G and G633A mutations, AMD1 was amplified with primer pairs 094E-F /094E-R and 094S-F /094S-R (Table S3), respectively. All the resulting mutant alleles of AMD1 were verified by sequencing and transformed into the amd1 mutant. Transformants of amd1 expressing the AMD1 WT-, AMD1 TGG -, PRP27 -AMD1 TGG -, and AMD1 TAA -GFP constructs were identified by PCR and examined for GFP signals by epifluorescence microscopy.
Specimen preparation for semi-thin sections
Perithecia collected from mating cultures at 8 or 10 dpf were fixed with 4% (vol/vol) glutaraldehyde in 0.1 M phosphate buffer (pH 6.8) overnight at 4 °C. Samples were then dehydrated in a series of acetone consisting of 30, 50, 70, 80, 90, and 100% (vol/vol). The dehydrated samples were embedded in Spurr resin as described33. Semi-thin sections (1 μm in thickness) were stained with 0.5% (wt/vol) toluidine blue before being examined with an Olympus BX-53 microscope.
qRT-PCR analysis
For qRT-PCR assays, RNA samples were isolated with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from perithecia collected at 7 dpf. The Fermentas First cDNA synthesis kit (Hanover, MD, USA) was used for cDNA synthesis. The TUB2 beta-tubulin gene was used as the internal control34 and the relative expression of each gene was calculated with the 2−△△Ct method. Data from three biological replicates were used to calculate the mean and standard deviation of the expression levels35.
Plant infection assays
For infection assays with flowering wheat heads of cultivar Xiaoyan 22, conidia were harvested from 5-day-old CMC cultures and re-suspended to 2.0 × 105 conidia/ml in sterile distilled water. For each head, the fifth spikelet from the base of the inflorescence was inoculated with 10 μl of conidium suspensions as described36, 37. FHB symptoms were examined at 14 day post-infection to estimate the disease index38, 39. Corn silks were infected with culture blocks and examined as described40.
RNA-seq analysis
Perithecia of PH-1 and amd1 mutant were harvested from carrot agar cultures at 7 dpf and used for RNA extraction with TRIzol (Invitrogen, USA). For each strain, RNA was isolated from two biological replicates. RNA-seq libraries were prepared with the NEBNext® Ultra™ Directional RNA Library Prep Kit (NEB, USA) following the instruction provided by the manufacturer and sequenced with Illumina HiSeq 2500 with the paired-end 2 × 150 bp model at the Novogene Bioinformatics Institute (Beijing, China). For each replicate, at least 24 Mb paired-end reads were obtained. The resulting RNA-seq reads were mapped onto the reference genome of F. graminearum strain PH-128, 41 by HISAT242. The number of reads (count) mapped to each gene were calculated by featureCounts43. Differential expression analysis of genes was performed using the edgeRun package44 with the exactTest function. Genes with a FDR (false discovery rate) of below 0.05 and |log2FC (log2 fold change)| of above 1 were regarded as differentially expressed genes.
Data availability
RNA-seq data were deposited at NCBI SRA database under accession number SRP100650.
References
Proctor, R. H., Hohn, T. M. & Mccormick, S. P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe In 8, 593–601 (1995).
Bai, G. H., Desjardins, A. E. & Plattner, R. D. deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 153, 91–98 (2002).
Bennett, R. J. & Turgeon, B. G. Fungal sex: the ascomycota. Microbiology Spectrum 4, 5 (2016).
Trail, F., Gaffoor, I. & Vogel, S. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fuarium graminearum). Fungal Genet Biol 42, 528–533 (2005).
Kim, H. K., Cho, E. J., Lee, S., Lee, Y. S. & Yun, S. H. Functional analyses of individual mating-type transcripts at MAT loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiol Lett 337, 89–96 (2012).
Zheng, Q. et al. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One 10, e0131623 (2015).
Kim, H. K. et al. A large-scale functional analysis of putative target genes of mating-type loci provides insight into the regulation of sexual development of the cereal pathogen Fusarium graminearum. PLoS genetics 11, e1005486 (2015).
Wang, C. et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathogens 7, e1002460 (2011).
Yun, Y. Z. et al. Functional analysis of the Fusarium graminearum phosphatome. New Phytologist 207, 119–134 (2015).
Son, H. et al. A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog 7, e1002310 (2011).
Son, H., Lee, J. & Lee, Y. W. A novel gene, GEA1, is required for ascus cell-wall development in the ascomycete fungus Fusarium graminearum. Microbiology 159, 1077–1085 (2013).
Liu, H. et al. Genome-wide A-to-I RNA editing in fungi independent of ADAR enzymes. Genome Research 26, 499–509 (2016).
Liu, H. et al. Two Cdc2 kinase genes with distinct functions in vegetative and infectious hyphae in Fusarium graminearum. Plos Pathogens 11, e1004913 (2015).
Zhao, Z. T. et al. Molecular evolution and functional divergence of tubulin superfamily in the fungal tree of life. Scientific Reports 4, 6746 (2014).
Bass, B. L. RNA editing by adenosine deaminases that act on RNA. Annual Review of Biochemistry 71, 817–846 (2002).
Cavinder, B., Sikhakolli, U., Fellows, K. M. & Trail, F. Sexual development and ascospore discharge in Fusarium graminearum. Jove-J Vis Exp 61, e3895 (2012).
Bruno, K. S., Tenjo, F., Li, L., Hamer, J. E. & Xu, J. R. Cellular localization and role of kinase activity of PMK1. In Magnaporthe grisea. Eukaryotic cell 3, 1525–1532 (2004).
Jiang, C. et al. FgSKN7 and FgATF1 have overlapping functions in ascosporogenesis, pathogenesis and stress responses in Fusarium graminearum. Environmental Microbiology 17, 1245–1260 (2015).
Luo, Y. et al. FgKin1 kinase localizes to the septal pore and plays a role in hyphal growth, ascospore germination, pathogenesis, and localization of Tub1 beta-tubulins in Fusarium graminearum. The New phytologist 204, 943–954 (2014).
Hibbett, D. S. et al. A higher-level phylogenetic classification of the Fungi. Mycol Res 111, 509–547 (2007).
Geiser, D. M. et al. Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98, 1053–1064 (2006).
Read, N. D. & Beckett, A. Ascus and ascospore morphogenesis. Mycological Research 100, 1281–1314 (1996).
Trail, F., Xu, H., Loranger, R. & Gadoury, D. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia 94, 181–189 (2002).
Trail, F. & Common, R. Perithecial development by Gibberella zeae: a light microscopy study. Mycologia 92, 130–138 (2000).
Tassan, J. P. & Le, G. X. An overview of the KIN1/PAR-1/MARK kinase family. Biology of the Cell 96, 193–199 (2004).
Min, K. et al. A novel gene, ROA, is required for normal morphogenesis and discharge of ascospores in Gibberella zeae. Eukaryot Cell 9, 1495–1503 (2010).
Son, H., Lee, J. & Lee, Y.-W. Mannitol induces the conversion of conidia to chlamydospore-like structures that confer enhanced tolerance to heat, drought, and UV in Gibberella zeae. Microbiological Research 167, 608–615 (2012).
Cuomo, C. A. et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317, 1400–1402 (2007).
Hou, Z. M. et al. A mitogen-activated protein kinase Gene (MGV1) in Fusarium graminearum is required for female fertility, Heterokaryon Formation, and Plant Infection. Mol Plant Microbe In 15, 1119–1127 (2002).
Zhou, X. Y., Heyer, C., Choi, Y. E., Mehrabi, R. & Xu, J. R. The CID1 cyclin C-like gene is important for plant infection in Fusarium graminearum. Fungal Genetics and Biology 47, 143–151 (2010).
Gao, X. et al. FgPrp4 kinase is important for spliceosome B-complex activation and splicing efficiency in Fusarium graminearum. PLoS genetics 12, e1005973 (2016).
Zhou, X., Li, G. & Xu, J. R. Efficient approaches for generating GFP fusion and epitope-tagging constructs in filamentous fungi. Methods in molecular biology 722, 199–212 (2011).
Cao, S. et al. FgSsn3 kinase, a component of the mediator complex, is important for sexual reproduction and pathogenesis in Fusarium graminearum. Scientific reports 6, 22333 (2016).
Jiang, C. et al. TRI6 and TRI10 play different roles in the regulation of deoxynivalenol (DON) production by cAMP signalling in Fusarium graminearum. Environmental Microbiology 18, 3689–3701 (2016).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Li, C. et al. FgCDC14 regulates cytokinesis, morphogenesis, and pathogenesis in Fusarium graminearum. Molecular microbiology 98, 770–786 (2015).
Lysoe, E., Seong, K. Y. & Kistler, H. C. The transcriptome of Fusarium graminearum during the infection of wheat. Mol Plant Microbe In 24, 995–1000 (2011).
Ding, S. L. et al. Transducin beta-like gene FTL1 is essential for pathogenesis in Fusarium graminearum. Eukaryotic cell 8, 867–876 (2009).
Howlett, B. J., Jonkers, W., Dong, Y., Broz, K. & Corby Kistler, H. The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum. PLoS Pathogens 8, e1002724 (2012).
Seong, K., Hou, Z. M., Tracy, M., Kistler, H. C. & Xu, J. R. Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum. Phytopathology 95, 744–750 (2005).
King, R., Urban, M., Hammond-Kosack, M. C., Hassani-Pak, K. & Hammond-Kosack, K. E. The completed genome sequence of the pathogenic ascomycete fungus Fusarium graminearum. BMC genomics 16, 544 (2015).
Kim, D., Landmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12, 357–360 (2015).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Dimont, E., Shi, J., Kirchner, R. & Hide, W. edgeRun: an R package for sensitive, functionally relevant differential expression discovery using an unconditional exact test. Bioinformatics 31, 2589–2590 (2015).
Acknowledgements
We thank Chaohui Li and Dr. Qinhu Wang for assistance with drawing ascocarp diagrams and GO enrichment figure. We also thank Dr. Cong Jiang for fruitful discussions. This work was supported by grants from the US Wheat and Barley Scab Initiative and National Science Fund for Excellent Young Scholars (no. 31622045).
Author information
Authors and Affiliations
Contributions
J.R.X. and H.L. conceived and designed the experiments, S.C., Y.H., C.H., Y.X., C.W., H.Z., and H.L. performed the experiments and data analyses. S.C., H.L., and J.R.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, S., He, Y., Hao, C. et al. RNA editing of the AMD1 gene is important for ascus maturation and ascospore discharge in Fusarium graminearum . Sci Rep 7, 4617 (2017). https://doi.org/10.1038/s41598-017-04960-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04960-7
This article is cited by
-
Fungal RNA editing: who, when, and why?
Applied Microbiology and Biotechnology (2020)
-
FgEaf6 regulates virulence, asexual/sexual development and conidial septation in Fusarium graminearum
Current Genetics (2020)
-
A-to-I mRNA editing in fungi: occurrence, function, and evolution
Cellular and Molecular Life Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.