Abstracts
Visual loss caused by retinopathy of prematurity (ROP) will be prevented if treatment-requiring ROP (TR-ROP) can be predicted. In this retrospective study including 418 infants with ≤32 weeks of gestational age (GA) and/or ≤1500 grams of birthweight, we attempted to identify useful predictors. We also examined the efficiency of significant predictors compared with existing predictive models, ROPScore and CHOP model. Multivariable logistic regression analyses supported the following factors were useful for predicting TR-ROP from all infants and infants with any ROP: GA (odds ratio [OR], 0.47 and 0.48), history of late-onset circulatory collapse (LCC) (OR, 2.76 and 2.44) and use of continuous positive airway pressure (CPAP) at 35 weeks of postmenstrual age (OR, 3.78 and 4.50). The comparison of areas under receiver operating characteristic curves indicated the combination of LCC, CPAP and ROPScore was better than ROPScore to predict TR-ROP from all infants and infants with any ROP (P = 0.007 and 0.02) and the combination of LCC, CPAP and CHOP model was also better than CHOP model to predict TR-ROP from all infants and infants with any ROP (P = 0.01 and 0.02). Our results suggested infants with a history of LCC and a long CPAP support have a high incidence of TR-ROP.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disorder that occurs in the retina of preterm infants. Due to the improvement of the mortality rate of immature babies, the number of infants with ROP have tended to increase worldwide1. Although existing therapies such as laser photocoagulation, vitrectomy and intravitreal injection of antibody specific for vascular endothelial growth factor have been shown to have certain effects for the attenuation of ROP progression2,3,4, ROP is still a leading cause of visual loss in childhood. Because the provision of the optimal treatment at the proper time is essential to prevent ROP progression, the identification of more useful predictive factors for treatment-requiring ROP (TR-ROP) that can be used at earlier stages after birth is urgently needed.
Although the details of the pathogenesis of ROP have not been revealed, it is quite certain that retinal hypoxia is the core of ROP development. Retinal hypoxia is closely related to neovascularization and vascular hyper-permeability after the arrest of physiological retinal vascular growth, and the retinal hypoxia eventually enhances the formation of fibrovascular membrane which causes tractional retinal detachment5, 6. Oxygen transport to the tissue is disturbed by circulatory and pulmonary dysfunctions. Among such dysfunctions, (1) a decrease in blood pressure and effective circulating blood volume by an infection and circulatory disorder, (2) an insufficiency of blood oxygenation due to respiratory disease, and (3) a reduction in oxygen transport ability caused by anemia are representative conditions that induce tissue hypoxia. Our purpose in conducting the present study was to identify useful predictors for the development of TR-ROP from the standpoint of retinal hypoxia.
Specifically, we listed late-onset circulatory collapse (LCC), sepsis, patent ductus arteriosus (PDA) requiring ligation, anemia requiring blood transfusion, use history of oxygen, use of continuous positive airway pressure (CPAP) and bronchopulmonary dysplasia (BPD) as indicators of circulatory and pulmonary dysfunctions which could occur in newborns. We also listed intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL) as candidate factors which could cause retinal hypoxia, because they reflect the circulatory disorder in the central nervous systems (CNS). The relationship of these factors to the onset of TR-ROP was investigated in this study.
Methods
This retrospective study was conducted under the approval of Institutional Review Board of Kyushu University Hospital and the principles of the Declaration of Helsinki. Informed consent regarding the use of data was obtained from each newborn’s parents.
Patients
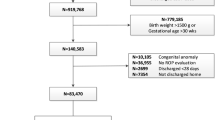
We analyzed the cases of neonates with ≤32 weeks of gestational age (GA) and/or ≤1500 grams of birthweight (BW) who were born at Kyushu University Hospital and underwent ophthalmic screening for ROP in a neonatal intensive care unit between November 2007 and November 2016. All infants were followed up until discharge at intervals indicated by ophthalmologists.
ROP classification
The staging of the infants’ ROP was decided according to International Classification of Retinopathy of Prematurity Revisited7. We categorized all infants into three groups: TR-ROP, mild ROP and no ROP. Treatments were performed for infants with ‘type 1 ROP’ as described in the Early Treatment for Retinopathy of Prematurity Randomized Trial (stage 2 or 3 in zone II with plus disease, stage 3 in zone I with or without plus disease, or stage 1 or 2 disease in zone I with plus disease)2 or worse ROP, and these infants were classified into the group of TR-ROP. Infants with ROP of the less activity than TR-ROP were classified into the group of mild ROP. The stages of ROP adopted for statistical analyses were determined based on the stage and zone at the time of the infant’s initial treatment in the cases of TR-ROP, and at the time that the ROP had advanced the most in the cases of mild ROP. The day when we provided TR-ROP with the initial treatment or when mild ROP advanced most was defined as the day of ROP maturation.
Predictive factors for TR-ROP
We investigated the following factors. GA, BW and sex were used as parameters of baseline at birth. As parameters of circulatory dysfunction, we examined LCC, sepsis, PDA requiring ligation, and blood transfusion before 35 weeks of postmenstrual age (PMA). LCC had been diagnosed by the review of medical records. Diagnostic criteria of LCC was summarized in Table 1. As parameters of pulmonary dysfunction, we examined the use history of oxygen, use of CPAP at 35 weeks of PMA, and BPD. The diagnosis of BPD was in accordance with described criteria8. We used IVH and PVL which occurred until 35 weeks of PMA as indicators of circulation in the CNS including the retina. We also examined the infants’ weight gain at 6 weeks after birth and the daily weight gain rate as parameters of growth after birth, which are used as parameters of existing predictive models, ROPScore and CHOP (Children’s Hospital of Philadelphia) model9, 10. The daily weight gain rate was calculated as described previously11. In brief, we divided the body weight change in one week from 8th to 15th postnatal day by 7 and defined this value as the daily weight gain rate in this study. We also confirmed whether significant predictors were beneficial to forecast TR-ROP by the comparison with ROPScore or CHOP model.
Statistical analyses
All analyses were carried out using SAS software (ver. 9.3, SAS, Cary NC). GA, BW, and weight gain at 6 weeks after birth were treated as continuous variables, and the other parameters were treated as categorical variables.
Because the current screening guideline for ROP uses only two factors of GA and BW which are recognized as universal risk factors for ROP1, 6, 12, 13, we calculated GA- and BW-adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs). For predictive factors that were marginally significant (P < 0.1), we calculated the ORs and 95% CIs in multivariable-adjusted logistic regression analyses. To assess the ability of each predictive factor for TR-ROP over a range of values, we also calculated the area under the receiver operating characteristic (ROC) curve and compared the areas under the ROC curves (AUCs)14, 15. The cutoff values were obtained from the maximum values of Youden index (sensitivity + specificity − 1)16. A two-sided P-value < 0.05 was considered significant.
Results
Characteristics of infants in this study
We enrolled 418 infants in this study. Their characteristics are summarized in Table 2. Among them, 76 infants (18.2%) developed TR-ROP, 119 infants (28.5%) developed mild ROP, and 223 infants (53.3%) didn’t developed any ROP. The mean (standard deviation [SD]) PMA of ROP maturation in TR-ROP infants and mild ROP infants was 35.5 (2.3) weeks and 35.6 (2.7) weeks, respectively.
Prediction of TR-ROP from all infants
Table 3 shows the results of our GA- and BW-adjusted and multivariable-adjusted logistic regression analyses of candidate factors to predict TR-ROP among all 418 infants. The results revealed that GA (OR, 0.47; 95% CI, 0.35–0.61; P < 0.0001), a history of LCC (OR, 2.76; 95% CI, 1.24–6.19; P = 0.01), and the use of CPAP at 35 weeks’ PMA (OR, 3.78; 95% CI, 1.66–8.93; P = 0.002) were independent significant predictors.
We next determined the ROC curves and calculated the AUCs to evaluate the utility of these factors (Supplementary Fig. 1, Table 4). The combination of GA and BW predicted TR-ROP among all infants with the AUC of 0.933 (95% CI, 0.904–0.963). When LCC and CPAP were added to GA and BW, the AUC became significantly larger (AUC, 0.950; 95% CI, 0.924–0.975; P = 0.006).
To confirm whether LCC and CPAP can be used to predict TR-ROP, we compared them with ROPScore and CHOP model, which are existing predictive models for ROP. ROPScore is based on GA, BW, weight gain at 6 weeks after birth, use history of oxygen, and blood transfusion10. CHOP model is based on GA, BW and weight gain rate9. We calculated the AUCs for ROPScore and CHOP model, and we found that they are not significantly larger than the AUC for the combination of GA and BW (P = 0.57 and 0.21, respectively). However, the combination of ROPScore, LCC and CPAP predicted TR-ROP more than both the combination of GA and BW and the ROPScore alone (AUC, 0.951; 95% CI, 0.926–0.976; P = 0.005 and 0.007, respectively). The combination of CHOP model, LCC and CPAP was also better than the combination of GA and BW and the CHOP model alone (AUC, 0.950; 95% CI, 0.925–0.975; P = 0.006 and 0.01, respectively). We additionally calculated the predicted probability of TR-ROP using the following equation: Probability of TR-ROP = 1/[1 + exp (−risk score)], where risk score = 18.738 + (−0.770) *GA + (−0.000824)*BW + 1.140*I (LCC) + 1.542*I (CPAP), where I (A) = 1 if A is present and 0 otherwise. Using the cutoff value of 0.252, the sensitivity was 92.9% and specificity was 89.1% for predicting TR-ROP from all infants.
Prediction of TR-ROP among the infants with any degree of ROP
We conducted GA- and BW-adjusted and multivariable-adjusted logistic regression analyses of candidate factors for predicting TR-ROP among the infants with any degree of ROP (Table 5). These results also proved that GA (OR, 0.48; 95% CI, 0.36–0.64; P < 0.0001), a history of LCC (OR, 2.44; 95% CI, 1.08–5.56; P = 0.03), and the use of CPAP at 35 weeks of PMA (OR, 4.50; 95% CI, 1.91–11.09; P = 0.0005) were significant factors.
Similarly, we drew ROC curves and calculated the AUCs (Supplementary Fig. 2, Table 6). The AUC for the combination of GA and BW to predict TR-ROP among infants with any degree of ROP was 0.859 (95% CI, 0.803–0.916). When LCC and CPAP were added to GA and BW, the AUC became significantly larger (AUC, 0.898; 95% CI, 0.851–0.945; P = 0.009).
To examine the efficiency of LCC and CPAP as predictors of TR-ROP, we compared them with ROPScore and CHOP model. Compared to the combination of GA and BW, the prediction of TR-ROP was not improved by the ROPScore (P = 0.40), but it was improved by the CHOP model (AUC, 0.865; 95% CI, 0.809–0.920; P = 0.03). The combination of ROPScore, LCC and CPAP predicted TR-ROP more than both the combination of GA and BW and the ROPScore alone (AUC, 0.898; 95% CI, 0.851–0.945; P = 0.009 and P = 0.02, respectively). The combination of CHOP model, LCC and CPAP was also better than the combination of GA and BW and the CHOP model alone (AUC, 0.900; 95% CI, 0.854–0.946; P = 0.007 and P = 0.02, respectively). We additionally calculated the predicted probability of TR-ROP using the following equation: Probability of TR-ROP = 1/[1 + exp (−risk score)], where risk score = 16.761 + (−0.718)*GA + 0.000159*BW + 1.088*I (LCC) + 1.701*I (CPAP), where I (A) = 1 if A is present and 0 otherwise. Using the cutoff value of 0.340, the sensitivity was 87.1% and specificity was 79.3% for predicting TR-ROP from infants with any degree of ROP.
Discussion
To identify predictors of the development of TR-ROP from the standpoint of retinal hypoxia, we chose parameters related to circulatory and pulmonary dysfunctions and circulatory disorders in the CNS in addition to parameters of the immaturity of physique and development. The mean PMA that infants received laser photocoagulation was 35.2 weeks in the Early Treatment for Retinopathy of Prematurity Randomized Trial17 and the mean PMA of ROP maturation in infants with TR-ROP was 35.5 weeks. Therefore, we set the deadline for judging whether parameters of LCC, sepsis, PDA ligation, transfusion, use history of oxygen, CPAP, IVH and PVL were positive to 35 weeks of PMA. The feature of this study to be emphasized most is that a history of LCC was included as the candidate factor for predicting TR-ROP.
LCC is defined as a late-onset circulatory collapse that occurs suddenly in newborns whose circulation dynamics have stabilized once after leaving the acute phase after birth18,19,20. The circulatory failure in LCC does not respond to volume expansion or vasopressors, but it does respond to an administration of glucocorticoid21. Because LCC appears particularly in infants with early GA and low BW19, 20, it has been speculated that the incidence of LCC will increase.
Previous studies revealed that the responses of cortisol and adrenocorticotropic hormone (ACTH) to stimulation by corticotropin-releasing hormone were lower in premature infants than in full-term infants, and the basal value of cortisol was higher in preterm infants than in full-term infants22, 23. In other words, preterm infants may have a weakened ability to upregulate cortisol in response to various stresses although the basal secretion is preserved. The basal value of cortisol after birth begins to decline from the initial 1 week22, and LCC frequently appears at the same time. Because the circulation dynamics are markedly stabilized by an administration of glucocorticoid, it is thought that relative adrenal insufficiency is one of the major causes of LCC19, 21, 24. However, the endocrine function of premature infants remains unclear in many respects, and there is no definite opinion regarding the appropriate range for cortisol and ACTH concentrations in preterm neonates25, 26. For these reasons, LCC is defined only as a syndrome with ‘late-onset’ circulatory failure without obvious causes which responds to glucocorticoids at the present stage.
Although the etiology of LCC has not been clarified, it was demonstrated that the CNS blood flow is decreased in LCC patients27, 28, and that infants with LCC have a high incidence of IVH, PVL and pulmonary dysfunction19, 20. Several studies have revealed that LCC has a close relationship with circulatory and pulmonary failures which are also recognized as risk factors of ROP6, 29, 30, but our results of multivariable logistic regression analyses showed that LCC was independently related to the severity of ROP. The present study is the first to demonstrate that LCC is a useful predictive factor for TR-ROP.
As described in previous reports19, 20, LCC tended to occur in infants with shorter GA and lower BW (Supplementary Table 1). LCC appeared prior to ROP maturation in all infants with both LCC and TR-ROP. However, contrary to our expectations LCC did not give any significant difference in the characteristics of population with TR-ROP (Supplementary Table 2). The reason remains unknown, and the further investigation about the influence of LCC on retinal blood flow is urgently needed.
In the present investigation, we also examined the relationship of respiratory assistance with CPAP to the development of TR-ROP. The use of CPAP can achieve a reduction of ventilation- and oxygen-mediated lung injury by shortening the duration of intubation and the use of oxygen. Moreover, various systems of non-invasive CPAP have been developed such as nasal CPAP (n-CPAP) and biphasic n-CPAP, which are well-established modes of respiratory support for preterm neonates31,32,33,34. Although pulmonary dysfunction has certainly decreased with the spread of CPAP, conditions that require respiratory support still indicate an immaturity of pulmonary function. However, no consensus has been obtained regarding whether the use of CPAP is associated with the onset of TR-ROP. Our present analyses confirmed that a long CPAP support can be used to predict the development of TR-ROP. We speculate possibilities as follows; (1) CPAP contributes to the reduction of lung injury, but the injury does not completely disappear and (2) long-term support with CPAP implies that respiratory complications such as apnea remain, indicating inefficiency of blood oxygenation that ultimately promotes retinal hypoxia. Although there are both positive and negative opinions regarding the relationship between CPAP use and ROP development35,36,37,38, it is possible that this disagreement is caused by difference in the characteristics of the studies’ patients. The current screening guideline for ROP is based on GA and BW which are recognized as universal risk factors for ROP worldwide1, 6, 12, 13. We therefore adjusted all parameters with GA and BW before conducting the statistical analyses, and the results revealed that long-term support with CPAP is a significant predictor of TR-ROP. However, a prospective and large-scale trial is needed before making any firm conclusions, of course.
Our findings also indicate that a history of LCC and a long CPAP support can be used to help predict TR-ROP, based on the AUC results. We observed that the model combining LCC, CPAP, GA and BW was significantly better than the model combining GA and BW for the prediction of TR-ROP both among all infants and among infants with any degree of ROP. We also calculated AUCs using the ROPScore and the CHOP model from among the various predictive models for ROP because their parameters could be extracted from our data base11, 39. The prediction of TR-ROP from all infants was not improved by using the ROPScore or CHOP model alone compared to the model using GA and BW. The prediction of TR-ROP from infants with any ROP was not improved by using the ROPScore, but it was improved by using CHOP model compared to the model using GA and BW. On the other hand, the prediction of TR-ROP both from all infants and from infants with any ROP was significantly improved by using the model combined with GA, BW, LCC and CPAP. The prediction was markedly improved by the addition of LCC and CPAP use to each model compared with the model using GA and BW. Surprisingly, either model combining LCC, CPAP and the ROPScore or CHOP model was better than the ROPScore or CHOP model alone. These results supported that LCC and CPAP have possibilities to become valuable indicators. However, we originally should update the value of weight gain rate and re-calculate the risk score on a weekly basis when using CHOP model. But in this study we divided the weight change from 8th to 15th postnatal day by 7 and used this value as weight gain rate as described previously11. This is the limitation of this investigation. To confirm whether this new predictive model combined with GA, BW, LCC and CPAP is better than existing clinical models, the prospective and multicenter study is required.
Another important point to be highlighted is that both LCC and CPAP use are easy to use as parameters. The current screening guideline recommends that the initial eye examination should be performed within 31 weeks of PMA or within 4 weeks after birth12. Considering that the mean (SD) PMA and postnatal day of LCC onset in our patient series were 29.3 (2.5) weeks and 22.3 (12.7) days (Supplementary Table 1), it is possible that we can obtain the crucial information before the initial examination. We can also determine at a glance whether CPAP support is continued or not. Because no complicated procedure is required, we speculate that ROP prediction using the two parameters of LCC and CPAP use will be beneficial for busy clinical ophthalmologists.
We identified useful predictive factors for TR-ROP. Our findings suggest that preterm infants with a history of LCC and long-term respiratory support by CPAP are at high risk of developing TR-ROP.
References
Shah, P. K. et al. Retinopathy of prematurity: Past, present and future. World J Clin Pediatr 5, 35–46 (2016).
Good, W. V. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 102, 233–248; discussion 248–250 (2004).
Karacorlu, M., Hocaoglu, M., Sayman Muslubas, I. & Arf, S. Long-term functional results following vitrectomy for advanced retinopathy of prematurity. Br J Ophthalmol, doi:10.1136/bjophthalmol-2016-309198 (2016).
Mintz-Hittner, H. A., Kennedy, K. A. & Chuang, A. Z. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364, 603–615 (2011).
Cavallaro, G. et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol 92, 2–20 (2014).
Hellstrom, A., Smith, L. E. & Dammann, O. Retinopathy of prematurity. Lancet 382, 1445–1457 (2013).
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 123, 991–999 (2005).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163, 1723–1729 (2001).
Binenbaum, G. et al. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch Ophthalmol 130, 1560–1565 (2012).
Eckert, G. U., Fortes Filho, J. B., Maia, M. & Procianoy, R. S. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond) 26, 400–406 (2012).
Piermarocchi, S. et al. Predictive algorithms for early detection of retinopathy of prematurity. Acta Ophthalmol 95, 158–164 (2017).
Chen, J., Stahl, A., Hellstrom, A. & Smith, L. E. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr 23, 173–178 (2011).
Fierson, W. M. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 131, 189–195 (2013).
Hanley, J. A. & McNeil, B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36 (1982).
Zweig, M. H. & Campbell, G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39, 561–577 (1993).
Youden, W. J. Index for rating diagnostic tests. Cancer 3, 32–35 (1950).
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 121, 1684–1694 (2003).
Kusuda, S. et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics 118, e1130–1138 (2006).
Miwa, M., Kusuda, S. & Ikeda, K. International Perspectives: Late-onset Circulatory Collapse in Very Low-birthweight Infants: A Japanese Perspective. NeoReviews 10, e381–e386 (2009).
Nakanishi, H. et al. Clinical characterization and long-term prognosis of neurological development in preterm infants with late-onset circulatory collapse. J Perinatol 30, 751–756 (2010).
Shimokaze, T., Saito, E. & Akaba, K. Increased Incidence of Late-Onset Circulatory Collapse after Changing Clinical Practice: A Retrospective Investigation of Causative Factors. Am J Perinatol 32, 1169–1176 (2015).
Ng, P. C. et al. A prospective longitudinal study to estimate the “adjusted cortisol percentile” in preterm infants. Pediatr Res 69, 511–516 (2011).
Niwa, F. et al. Limited response to CRH stimulation tests at 2 weeks of age in preterm infants born at less than 30 weeks of gestational age. Clin Endocrinol (Oxf) 78, 724–729 (2013).
Masumoto, K. et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res 63, 686–690 (2008).
Ng, P. C. Is there a “normal” range of serum cortisol concentration for preterm infants? Pediatrics 122, 873–875 (2008).
Sari, F. N. et al. Baseline and stimulated cortisol levels in preterm infants: is there any clinical relevance? Horm Res Paediatr 77, 12–18 (2012).
Fukuda, S. et al. Late circulatory dysfunction and decreased cerebral blood flow volume in infants with periventricular leukomalacia. Brain Dev 30, 589–594 (2008).
Washio, Y. et al. Hemodynamic analysis in infants with late-onset circulatory collapse. Pediatr Int 55, 582–588 (2013).
Port, A. D., Chan, R. V., Ostmo, S., Choi, D. & Chiang, M. F. Risk factors for retinopathy of prematurity: insights from outlier infants. Graefes Arch Clin Exp Ophthalmol 252, 1669–1677 (2014).
Watts, P., Adams, G. G., Thomas, R. M. & Bunce, C. Intraventricular haemorrhage and stage 3 retinopathy of prematurity. Br J Ophthalmol 84, 596–599 (2000).
Finer, N. N. et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 362, 1970–1979 (2010).
Geary, C., Caskey, M., Fonseca, R. & Malloy, M. Decreased incidence of bronchopulmonary dysplasia after early management changes, including surfactant and nasal continuous positive airway pressure treatment at delivery, lowered oxygen saturation goals, and early amino acid administration: a historical cohort study. Pediatrics 121, 89–96 (2008).
Kribs, A. et al. Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr 169, 723–730 (2015).
O’Brien, K., Campbell, C., Brown, L., Wenger, L. & Shah, V. Infant flow biphasic nasal continuous positive airway pressure (BP-NCPAP) vs. infant flow NCPAP for the facilitation of extubation in infants’ ≤1,250 grams: a randomized controlled trial. BMC Pediatr 12, 43, doi:10.1186/1471-2431-12-43 (2012).
Chen, Y. et al. Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing, China. Br J Ophthalmol 92, 326–330 (2008).
Hakeem, A. H., Mohamed, G. B. & Othman, M. F. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol 19, 289–294 (2012).
Ni, Y. Q. et al. Natural involution of acute retinopathy of prematurity not requiring treatment: factors associated with the time course of involution. Invest Ophthalmol Vis Sci 55, 3165–3170 (2014).
Raghu, R., Fisher, M., Cerone, J. & Barry, G. Non-invasive Respiratory Support and Severe Retinopathy of Prematurity. J Pediatr Ophthalmol Strabismus 53, e47–50 (2016).
Hutchinson, A. K. et al. Clinical Models and Algorithms for the Prediction of Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology. Ophthalmology 123, 804–816 (2016).
Acknowledgements
We thank Dr. Shunji Hikino and Dr. Terumichi Matsuo (Department of Pediatrics, National Kyushu Medical Center) for giving us the latest information about the management of circulatory and pulmonary disorders in newborns. This work was supported by MEXT KAKENHI Grant Number JP26293373. The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
M.A. designed this study. M.A., S.T., M.M. and K. Fujikawa collected the data. M.A., S.T. and K. Fujiwara performed data analyses. M.A. and K.H.S. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arima, M., Tsukamoto, S., Fujiwara, K. et al. Late-onset Circulatory Collapse and Continuous Positive Airway Pressure are Useful Predictors of Treatment-requiring Retinopathy of Prematurity: A 9-year Retrospective Analysis. Sci Rep 7, 3904 (2017). https://doi.org/10.1038/s41598-017-04269-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04269-5
This article is cited by
-
Long use of continuous positive airway pressure protects against the development of treatment-requiring retinopathy of prematurity
Scientific Reports (2022)
-
The association of various obstetric and perinatal factors with retinopathy of prematurity
International Ophthalmology (2022)
-
Retinopathy of prematurity in Rwanda: a prospective multi-centre study following introduction of screening and treatment services
Eye (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.