Abstract
Although preterm infant mortality is low, the proportion of patients with treatment-requiring retinopathy of prematurity (TR-ROP) is high in Japan. Various multicenter studies have reported the risk factors for TR-ROP; however, no large-scale studies have been conducted in Japan. We retrospectively analyzed 13,645 infants born at < 28 weeks’ gestation (January 1, 2009–December 31, 2018), and registered in the Neonatal Research Network of Japan database. TR-ROP was defined as ROP requiring retinal laser photocoagulation and/or intravitreal anti-vasoendothelial growth factor drugs. Multivariable logistic regression analysis was performed to identify factors associated with TR-ROP development. The median gestational age of enrolled infants was 26 weeks (interquartile range [IQR], 24–27 weeks), median birth weight was 760 g (IQR, 620–918 g). Proportion of patients with TR-ROP was 30.3%. TR-ROP was significantly associated with birth at < 26 weeks’ gestational age (adjusted odds ratio [aOR] 1.54), blood transfusion (aOR 1.49), invasive ventilation ≥ 28 days (aOR 1.41), sepsis (aOR 1.29), birth weight < 750 g (aOR 1.28), intraventricular hemorrhage (aOR 1.33), delayed achievement of full enteral feeding > 14 days (aOR 1.28), and continuous positive airway pressure (CPAP) therapy ≥ 28 days (aOR 0.79). Supplemental oxygen ≥ 28 days was not associated with TR-ROP development. Lower gestational age at birth and birth weight, blood transfusion, prolonged invasive ventilation, sepsis, intraventricular hemorrhage, and delayed achievement of full enteral feeding were risk factors for TR-ROP, whereas CPAP use was protective against TR-ROP.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a major neonatal morbidity and the most common avoidable cause of childhood blindness in middle-income countries1. In 2010, approximately 170,000 preterm infants developed ROP, with 54,000 of them requiring treatment worldwide2. According to North American statistics, 89.4% (1202/1344) of preterm infants born at ≤ 28 weeks of gestation and weighing ≤ 750 g developed ROP in at least one eye. By comparison, only 6.3% (33/529) of preterm infants born at ≥ 30 weeks of gestation with a birth weight of ≥ 1500 g developed ROP3. Therefore, among preterm infants, those born at an extremely low gestational age (before 28 weeks of gestation) are at a higher risk of developing ROP. Regarding the prevalence of treatment-requiring ROP (TR-ROP), the International Network for Evaluating Outcomes (iNeo) reported that 24.9% of preterm infants born between 24 and 28 weeks of gestation (2007–2013) required treatment for ROP. In their report, Japan had the highest survival rate (92%) but also the highest proportion of patients with TR-ROP (30.4%) among the participating countries4. Notably, a British nationwide population-based study reported that the proportion of patients with TR-ROP was 2.5 times higher in the recent years, than previously estimated5. The steady increase in the proportion of patients with TR-ROP is thought to be due to the increase in the survival rate of extremely preterm infants6.
Although genetic predisposition is thought to be involved in ROP7, the major monogenic mutations involved in the onset and severity of ROP have not been identified. In the United States, severe ROP has been reported to occur more frequently in Caucasian than in African-American infants, suggesting the presence of racial risk variation in ROP8. However, this racial difference might be explained by racial variation in socioeconomic status9. Until now, various multicenter studies have been conducted worldwide, including in the United States10,11 and Europe12,13, on the risk factors and treatment of ROP. However, few large-scale studies have been conducted in the Asian population. The main risk factors of ROP include the two main risk factors of prematurity (lower gestational age and birth weight), and oxygen supplementation14,15; several other factors such as intraventricular hemorrhage (IVH), sepsis, necrotizing enterocolitis (NEC), respiratory distress syndrome (RDS), blood transfusion, mechanical ventilation, failure to gain weight, treatment-requiring patent ductus arteriosus (PDA), thrombocytopenia16, antenatal corticosteroid17, and erythropoietin therapy9, have also been reported. Regarding the risk of oxygen supplementation, several reports have stated that the proportion of patients with ROP and its treatment rates have decreased significantly since the introduction of lower oxygen saturation targets in the acute phase owing to the introduction of pulse oximetry in the neonatal intensive care unit (NICU)18. In the findings of the NeOProM study, a meta-analysis of all the trials, low oxygen targets (85–89%) were associated with an increased incidence of necrotizing enterocolitis and death, but a reduced proportion of patients with TR-ROP19. While neonatal management that refrains from excessive oxygen use has become established, there is no large-scale study examining the relationship between oxygen supplementation and ROP in Japan.
In this study, risk factors for TR-ROP in Japan between 2009 and 2018 were investigated using the Neonatal Research Network of Japan (NRNJ) database. This is a prospective database consisting of 218 institutions that register the clinical information of newborns weighing ≤ 1500 g at birth or of those born at a gestational age of < 32 weeks.
Results
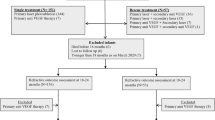
A total of 18,160 newborns were born at 22–27 weeks of gestation and/or had a birth weight of < 1500 g, between January 1, 2009 and December 31, 2018. Of these infants, 6617 were excluded due to the following reasons: death before discharge (2000 infants), major congenital anomalies (736 infants), transfer to other hospitals (1419 infants), and insufficient data regarding the diagnosis of ROP (2462 infants). Among the remaining 13,645 infants, 7041 (51.6%) eventually developed ROP with 4136 (30.3%) requiring treatment (TR-ROP) (Supplementary Fig. S1). The trends in the proportion of patients with TR-ROP and non-TR-ROP did not change significantly through the study period (Supplementary Fig. S2). There was a slight but significant decreasing trend in the gestational age of newborns screened for ROP (p < 0.01, Supplementary Fig. S3).
The clinical characteristics of infants with ROP are shown in Table 1. These infants were born at a median gestational age of 26 weeks (IQR, 24–27 weeks) and had a median birth weight of 760 g (IQR, 620–918 g). Overall, the proportion of infants with TR-ROP was 30.3%. Comparing the characteristics of infants in the TR-ROP and non-TR-ROP (n = 9509) groups, gestational age [TR-ROP vs. non-TR-ROP: 25 (24–26) vs. 26 (25–27) weeks], birth weight [674 (565–814) vs. 806 (658–954) g], and Apgar scores at 5 min [6 (5–8) vs. 7 (6–8)] were significantly lower, multiple births (18.0 vs. 16.2%) was significantly more common in the TR-ROP group (all p < 0.01). Regarding morbidities, the proportion of patients with RDS (84.1 vs. 78.8%), sepsis (18.2 vs. 10.3%), NEC (3.0 vs. 1.6%), IVH (31.2 vs. 19.1%), and treated PDA (60.5 vs. 55.9%) were significantly higher in the TR-ROP group (all p < 0.01). Regarding neonatal management, blood transfusion (80.6 vs. 58.0%), erythropoietin for anemia (93.8 vs. 92.3%), invasive ventilation (46 vs. 30 days), supplemental oxygen (85 vs. 66 days), oxygen use at 36 weeks of corrected age (68.5 vs. 64.6%), and parenteral nutrition (93.8 vs. 91.4%) were significantly more common, time from birth to full enteral feeding (15 vs. 13 weeks) was significantly longer, and use of CPAP (29 vs. 32 days) was significantly lower in the TR-ROP group compared to the non-TR-ROP group.
To determine the risk factors of TR-ROP, we performed multivariable logistic regression analysis (Table 2). TR-ROP was significantly associated with birth at a gestational age of < 26 weeks (adjusted odds ratio [aOR] 1.80, 99% CI 1.58–20.5), blood transfusion (aOR 1.57, 99% CI 1.37–1.79), invasive ventilation ≥ 28 days (aOR 1.50, 99% CI 1.28–1.70), sepsis (aOR 1.35, 99% CI 1.16–1.57), birth weight < 750 g (aOR 1.46, 99% CI 1.30–1.66), IVH (aOR 1.33, 99% CI 1.18–1.50), delayed achievement of full enteral feeding > 14 days (aOR 1.28, 99% CI 1.15–1.43), and CPAP ≥ 28 days (aOR 0.84, 99% CI 0.75–0.94) (all p < 0.001). Supplemental oxygen ≥ 28 days was not associated with the development of TR-ROP.
Discussion
This study identified several risk factors for TR-ROP in Japan from 2009 to 2018, including lower gestational age and birth weight, blood transfusion, prolonged invasive ventilation, sepsis, IVH, and delayed achievement of full enteral feeding; in contrast, the use of CPAP was protective against the development of TR-ROP. Prolonged oxygen exposure, which has been reported to be a major risk factor for ROP, was not an independent risk factor of ROP in this study.
To date, there have been few population-based reports examining the proportion of patients with and the risk factors of TR-ROP in the Asian population. In Taiwan, it was reported that the proportion of patients with TR-ROP was 6.5% (266/4096) among ROP patients born weighing ≤ 2000 g or born at a gestational age ≤ 32 weeks, based on a nationwide retrospective cross-sectional study from 2002 to 201120. In another study from Taiwan, it has been reported that male sex, birth weight < 1250 g, RDS, and PDA are risk factors of TR-ROP; however, the variables used for multivariable analysis in this study are unknown due to the publication form of conference proceedings21. In Korea, it was reported that the proportion of patients with TR-ROP was 3.0% (1246/42,300) among all ROP patients and 25.0% (561/2240) among ROP patients born at < 28 weeks of gestation, based on a nationwide epidemiological study from 2007 to 201822. The proportion of patients with TR-ROP in our study [(30.3% (4136/13,645)] was very close to the Japanese data reported by the iNeo study (30.4%)23, and was higher than that reported in the aforementioned Asian studies. Thus, it is likely that compared to other countries, ophthalmologists in Japan treat infants at an earlier stage of ROP23. Additionally, in recent times, anti-vasoendothelial growth factor (anti-VEGF) antibody treatment has become widespread in many facilities in Japan; this can be seen in the data captured throughout the length of this study (9.2% [380/4136 cases], Supplementary Fig. S4), and thus treatment for ROP may be increasing due to the convenience of this treatment.
In this study, we have clarified that gestational age < 26 weeks, blood transfusion, invasive ventilation ≥ 28 days, sepsis, and birth weight < 750 g are risk factors for TR-ROP, while the use of CPAP is a protective factor for TR-ROP in Japan. To date, only two large scale studies have investigated the risk factors for TR-ROP. Slidsborg et al., performed a retrospective register-based cohort study including 6490 preterm infants born at < 32 gestational weeks in Denmark from 1997 to 2008, and reported that the proportion of patients with TR-ROP was 4% (192/6490). In their study, gestational age at delivery, small for gestational age, male sex, mechanical ventilation, and blood transfusion were independent risk factors for TR-ROP among 31 known risk factors24. In the United States, Gonski et al., performed a database study involving 2306 TR-ROP infants out of 75,821 infants born weighing ≤ 1500 g or born at a gestational age ≤ 30 weeks, who were screened for ROP between 2006–2015; they reported that lower birth weight, mechanical ventilation on postnatal day 28, male sex, IVH, bacteremia, increased FiO2 on postnatal day 28, lack of antenatal steroids, white race, change in weight Z-score of ≥ 0, and NEC were independent risk factors for TR-ROP25. The independent risk factors for TR-ROP in our study (lower gestational age, lower birth weight, and duration of artificial ventilation) were generally consistent with those of two previous major reports24,25. In addition, blood transfusion was consistent with the former report and sepsis with the latter. The duration of CPAP was found to be an independent protective factor for TR-ROP in this study; this is a novel finding that has not been previously reported. Additionally, supplemental oxygen for ≥ 28 days, which has been reported as a major risk factor for ROP, was not a risk factor in this study. In high-income countries with adequate resources, where oxygen saturation is well controlled, oxygen administration is no longer reported as a risk for TR-ROP. However, multicenter studies from resource-limited countries such as Rwanda26 and China27 still suggest that oxygen administration is a risk factor for TR-ROP in low- and middle-income countries.
Regarding the ventilation strategy, Slidsborg et al., hypothesized that longer use of mechanical ventilation might worsen ROP through high-pressure oxygen and fluctuating oxygen levels24. Interestingly, in our study, a long duration of invasive ventilation was a risk factor for TR-ROP, while the duration of CPAP was protective. Similarly, a group at the University of Viena reported a higher rate of early CPAP treatment at birth (45–86% vs. 37–63%) and a lower proportion of patients with severe ROP (1–10% vs. 8–12%) than those of the Vermont Oxford Neonatal Network. They speculated that the lower rates of ROP in their institution might be related to the early initiation of CPAP28. However, the long-term advantage of CPAP has not been fully elucidated until now. A multicenter longitudinal follow-up study in Australia clarified that increased duration of assisted ventilation does not improve the rate of oxygen dependence at 36 weeks or lung function in childhood29. In addition, Nakashima et al., reported that the duration of CPAP use in NICUs in Japan has increased in recent years; however, the rate of bronchopulmonary dysplasia has increased in survivors between 2003 and 201630. In Japan, mechanical ventilation is the most common respiratory strategy for preterm infants with respiratory distress within the first 48 h of life, which is clearly different from other countries that prioritize CPAP31.
The effect of CPAP on the development of TR-ROP has not been fully investigated in previous studies. In a study in Denmark, CPAP therapy was identified as a risk factor for TR-ROP in univariate analysis (OR 1.86 [1.17–3.31]); however, their study only divided the study population into two groups based on the presence or absence of CPAP use; they did not consider the duration of CPAP like we did in our study24. In a study from the United States, CPAP was not investigated as an independent variable but as part of respiratory support in univariate analysis, and the proportion of patients on CPAP support on day 28 was similar between the TR-ROP and non-TR-ROP groups (12% vs. 10%)25. Similarly, in a single-center study in Japan, use of CPAP at 35 weeks of postmenstrual age was reported as an independent factor for predicting TR-ROP among infants with ROP (OR 4.50)32. Unlike these previous reports, our study identified the duration of CPAP as a protective factor for TR-ROP. The main difference between our study and previous ones is that we examined the duration of CPAP as a continuous variable, while previous studies examined the presence or absence of CPAP use at specific time points as a nominal variable. A limitation of all risk-factor studies to date is that their data have not been analyzed using survival analysis, which takes into account both the baseline clinical characteristics of the newborns and the changes that occur while in the NICU. We believe that it is better to analyze the “duration” of CPAP rather than its “use at a specific timepoint”, and the duration of ventilation.
Intriguingly, the duration of ventilation and CPAP were independent risk and protective factors of TR-ROP, respectively. We hypothesize that CPAP may mediate its protective effect by two possible mechanisms. First, CPAP improves oxygenation by reducing lung damage and increasing functional residual capacity33; it stabilizes systemic oxygenation to prevent retinal hypoxia and avoid the need for high-concentration oxygen therapy. Second, since the frequency of desaturation within 8 weeks of gestation and that of severe ROP are significantly associated in preterm infants born at < 28 weeks gestation34, CPAP use post-extubation, which reduces apnea and desaturation events associated with fluctuations of oxygen saturation35, might protect from TR-ROP. Thus, we hypothesized that the proportion of patients with TR-ROP could be reduced if artificial ventilation can be successfully switched to CPAP.
There are several limitations in this study. First, due to the retrospective nature of the study, there is insufficient information on the stage and treatment of ROP and the details regarding sepsis, if it was early or late. Thus, we could not perform detailed analysis according to disease severity. Second, in Japan, there might be inter-facility differences in policies for ROP treatment (including the initial time of examination, frequency of examination, and the threshold for treatment) and for respiratory support strategies such as oxygen saturation targets and the time to switch from mechanical ventilation to CPAP. For ROP screening and treatment, it is desirable to use guidelines that are standardized across the globe. In addition, no data on noninvasive ventilation other than CPAP, such as high-flow oxygen therapy, was available despite the fact that the use of these treatments has increased in recent years. In addition, there is a possibility that infants with more severe RDS were ventilated while those in whom RDS was less severe were given CPAP; this might be a residual confounding factor in this retrospective study. Finally, the status of thrombocytopenia16, antenatal corticosteroid17, and failure to gain weight36, which are now considered as risk factors for ROP, should be analyzed in future studies. Therefore, the protective effect of CPAP use on the development of TR-ROP needs to be verified by future prospective studies, with a clear definition of TR-ROP.
In this nationwide survey, we found that lower gestational age and birth weight, blood transfusion, prolonged invasive ventilation, and sepsis were risk factors for TR-ROP; however, the use of CPAP was protective against the development of TR-ROP.
Methods
Patients and samples
This study was approved by the ethics committee of the Kobe University Graduate School of Medicine (approval number: B210042). All data were fully anonymized before they were accessed by any of the authors.
We retrospectively analyzed infants who were born alive between January 1, 2009, and December 31, 2018, and registered in the NRNJ, which is the same database as that used in the iNeo study. NRNJ prospectively registers the clinical information of all infants who are born weighing ≤ 1500 g or at a gestational age of < 32 weeks and admitted to the 218 participating NICUs (accounting for 53.8% of the 405 secondary- and tertiary-level NICUs in Japan, including Kobe University Hospital) as previously published30. In general, the data on each infant is entered in the database once, at discharge. The inclusion criteria were as follows: infants who were born between 22 and 27 weeks of gestation (< 28 weeks) and observed from birth to discharge in the same institute. We excluded infants who died before discharge, who had major anomalies or congenital diseases, who had been transferred to another hospital, and who had no available records regarding the diagnosis of ROP.
Perinatal factors and TR-ROP
TR-ROP was defined as ROP requiring retinal laser photocoagulation and/or intravitreal anti-VEGF drugs37. The diagnosis and treatment of ROP was at the discretion of the attending ophthalmologist at each institution. The gestational age was calculated based on the results of an ultrasound examination in early pregnancy and the date of the last menstrual period. RDS was diagnosed by clinical and radiographic findings. Sepsis was defined as symptomatic culture-proven septicemia or bacteremia. Treated PDA was defined as PDA diagnosed based on both echocardiographic and clinical findings, and requiring medical or surgical treatment. NEC and IVH were both diagnosed by clinical and radiographic findings38. Invasive ventilation was defined as mechanical or high-frequency ventilation via an endotracheal tube, and CPAP included bilevel or continuous positive airway pressure equipped with a nasal mask or prongs according to previous publications30. Time to achieve full enteral feeding was defined as the number of days taken to first reach 100 ml/kg/day of enteral feeding. Parenteral nutrition was defined as use of intravenous hyperalimentation39.
Statistical analyses
Data were described as median (IQR) or numbers (percentages). Missing data were excluded from the analysis as was done in similar bronchopulmonary dysplasia studies that used the same database30. The Mann–Whitney nonparametric rank, Fisher's exact, and Chi-square tests were used to compare the two groups. Chi-square test for trend or one-way analysis of variance test were used to assess the trend throughout the study period. Based on a previously published evidence9, a multivariable logistic regression analysis was performed to identify the factors associated with the development of TR-ROP. Associations among the following variables and TR-ROP were assessed: gestational age, birth weight, male sex, multiple births, Apgar score, RDS, sepsis, NEC, IVH, treated PDA, blood transfusion, erythropoietin for anemia, invasive ventilation, CPAP, supplemental oxygen, duration from birth to full enteral feeding, and parenteral nutrition. We included all variables in the regression model after transforming continuous variables into binary variables, including gestational age < 26 weeks, invasive ventilation ≥ 28 days, birth weight < 750 g, delayed achievement of full enteral feeding (> 14 days), supplemental oxygen ≥ 28 days, and CPAP ≥ 28 days. Adjusted values were obtained from a logistic regression analysis after adjusting for male sex, multiple births, Apgar score of < 4 points at 5 min, treated PDA, and all variables listed in Table 2. A P value of < 0.01 was considered statistically significant. Data were expressed as median (interquartile range), number (%), and odds ratio (99% confidence interval [CI]).
Statistical analyses were performed using JMP 15.0 (SAS Institute Inc., Cary, NC, USA), GraphPad Prism version 7.00 (GraphPad Software, La Jolla, CA, USA), and SPSS (version 22.0; IBM Corp., Armonk, NY, USA).
The study protocol was performed in accordance with the relevant guidelines and with the approval of the Ethics Committee at Kobe University Graduate School of Medicine (IRB approval number: B210042, 16/June/2021). Since this is a retrospective data analysis that does not use patient specimens and does not involve any physical contact with patients, we obtained permission from the committee and substituted individual consent with an opt-out statement on the website of Kobe University Hospital.
Data availability
The data that support the findings of this study are available from NRNJ but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of NRNJ.
References
Gilbert, C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 84, 77–82. https://doi.org/10.1016/j.earlhumdev.2007.11.009 (2008).
Blencowe, H., Lawn, J. E., Vazquez, T., Fielder, A. & Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 74(Suppl 1), 35–49. https://doi.org/10.1038/pr.2013.205 (2013).
Quinn, G. E. et al. Incidence and early course of retinopathy of prematurity: Secondary analysis of the postnatal growth and retinopathy of prematurity (G-ROP) study. JAMA Ophthalmol. 136, 1383–1389. https://doi.org/10.1001/jamaophthalmol.2018.4290 (2018).
Darlow, B. A. et al. International variations and trends in the treatment for retinopathy of prematurity. Br. J. Ophthalmol. 101, 1399–1404. https://doi.org/10.1136/bjophthalmol-2016-310041 (2017).
Adams, G. G. et al. Treatment trends for retinopathy of prematurity in the UK: Active surveillance study of infants at risk. BMJ Open 7, e013366. https://doi.org/10.1136/bmjopen-2016-013366 (2017).
Tavassoli, S., Wach, R., Haynes, R., Markham, R. & Williams, C. Estimate of incidence of ROP requiring treatment in extreme preterms and impact on service-7 year review in tertiary unit. Eye (Lond) 33, 845–849. https://doi.org/10.1038/s41433-018-0330-x (2019).
Bizzarro, M. J. et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics 118, 1858–1863. https://doi.org/10.1542/peds.2006-1088 (2006).
Saunders, R. A. et al. Racial variation in retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch. Ophthalmol. 115, 604–608. https://doi.org/10.1001/archopht.1997.01100150606005 (1997).
Kim, S. J. et al. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 63, 618–637. https://doi.org/10.1016/j.survophthal.2018.04.002 (2018).
Schaffer, D. B. et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 100, 230–237. https://doi.org/10.1016/s0161-6420(93)31665-9 (1993).
Good, W. V., Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 102, 233–248 (2004) (discussion 248–250).
Zin, A. & Gole, G. A. Retinopathy of prematurity-incidence today. Clin. Perinatol. 40, 185–200. https://doi.org/10.1016/j.clp.2013.02.001 (2013).
Larsen, P. P. et al. Incidence of retinopathy of prematurity in Germany: Evaluation of current screening criteria. Arch. Dis. Child Fetal Neonatal Ed. 106, 189–193. https://doi.org/10.1136/archdischild-2020-319767 (2021).
Shah, V. A., Yeo, C. L., Ling, Y. L. & Ho, L. Y. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann. Acad. Med. Singap. 34, 169–178 (2005).
Fortes Filho, J. B. et al. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP). Graefes Arch. Clin. Exp. Ophthalmol. 248, 893–900. https://doi.org/10.1007/s00417-009-1248-6 (2010).
Parrozzani, R. et al. Severe retinopathy of prematurity is associated with early post-natal low platelet count. Sci. Rep. 11, 891. https://doi.org/10.1038/s41598-020-79535-0 (2021).
Yim, C. L. et al. Association of antenatal steroid and risk of retinopathy of prematurity: A systematic review and meta-analysis. Br. J. Ophthalmol. 102, 1336–1341. https://doi.org/10.1136/bjophthalmol-2017-311576 (2018).
Tin, W., Milligan, D. W., Pennefather, P. & Hey, E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch. Dis. Child Fetal Neonatal Ed. 84, F106-110. https://doi.org/10.1136/fn.84.2.f106 (2001).
Askie, L. M. et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA 319, 2190–2201. https://doi.org/10.1001/jama.2018.5725 (2018).
Kang, E. Y. et al. Ten-year epidemiology of retinopathy of prematurity treatment in Taiwan. Retina 40, 1804–1811. https://doi.org/10.1097/IAE.0000000000002684 (2020).
Wu, W.-C., Kang, Y.-C., Chu, S.-M. & Lien, R. A ten-year epidemiology of retinopathy of prematurity treatment in Taiwan. Investig. Ophthalmol. Vis. Sci. 60, 6515 (2019).
Hong, E. H. et al. Nationwide incidence and treatment pattern of retinopathy of prematurity in South Korea using the 2007–2018 national health insurance claims data. Sci. Rep. 11, 1451. https://doi.org/10.1038/s41598-021-80989-z (2021).
Darlow, B. A. et al. Variations in oxygen saturation targeting, and retinopathy of prematurity screening and treatment criteria in neonatal intensive care units: An international survey. Neonatology 114, 323–331. https://doi.org/10.1159/000490372 (2018).
Slidsborg, C. et al. Neonatal risk factors for treatment-demanding retinopathy of prematurity: A Danish national study. Ophthalmology 123, 796–803. https://doi.org/10.1016/j.ophtha.2015.12.019 (2016).
Gonski, S. et al. Risk of development of treated retinopathy of prematurity in very low birth weight infants. J. Perinatol. 39, 1562–1568. https://doi.org/10.1038/s41372-019-0487-6 (2019).
Mutangana, F. et al. Retinopathy of prematurity in Rwanda: A prospective multi-centre study following introduction of screening and treatment services. Eye (Lond) 34, 847–856. https://doi.org/10.1038/s41433-019-0529-5 (2020).
Dai, Y. et al. Incidence of retinopathy of prematurity treatment in extremely preterm infants in China. Paediatr. Perinat. Epidemiol. https://doi.org/10.1111/ppe.12810 (2021).
Kirchner, L. et al. Is the use of early nasal CPAP associated with lower rates of chronic lung disease and retinopathy of prematurity? Nine years of experience with the Vermont Oxford Neonatal Network. J. Perinat. Med. 33, 60–66. https://doi.org/10.1515/JPM.2005.010 (2005).
Doyle, L. W. et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N. Engl. J. Med. 377, 329–337. https://doi.org/10.1056/NEJMoa1700827 (2017).
Nakashima, T. et al. Trends in bronchopulmonary dysplasia among extremely preterm infants in Japan, 2003–2016. J. Pediatr. 230, 119-125 e117. https://doi.org/10.1016/j.jpeds.2020.11.041 (2021).
Beltempo, M. et al. Respiratory management of extremely preterm infants: An international survey. Neonatology 114, 28–36. https://doi.org/10.1159/000487987 (2018).
Arima, M. et al. Late-onset circulatory collapse and continuous positive airway pressure are useful predictors of treatment-requiring retinopathy of prematurity: A 9-year retrospective analysis. Sci. Rep. 7, 3904. https://doi.org/10.1038/s41598-017-04269-5 (2017).
Richardson, C. P. & Jung, A. L. Effects of continuous positive airway pressure on pulmonary function and blood gases of infants with respiratory distress syndrome. Pediatr. Res. 12, 771–774. https://doi.org/10.1203/00006450-197807000-00006 (1978).
Di Fiore, J. M. et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157, 69–73. https://doi.org/10.1016/j.jpeds.2010.01.046 (2010).
Miller, M. J., Carlo, W. A. & Martin, R. J. Continuous positive airway pressure selectively reduces obstructive apnea in preterm infants. J. Pediatr. 106, 91–94. https://doi.org/10.1016/s0022-3476(85)80475-3 (1985).
Shiraki, A. et al. Retrospective validation of the postnatal growth and retinopathy of prematurity (G-ROP) criteria in a Japanese cohort. Am. J. Ophthalmol. 205, 50–53. https://doi.org/10.1016/j.ajo.2019.03.027 (2019).
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 121, 1684–1694. https://doi.org/10.1001/archopht.121.12.1684 (2003).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7. https://doi.org/10.1097/00000658-197801000-00001 (1978).
Morisaki, N. et al. Brief parenteral nutrition accelerates weight gain, head growth even in healthy VLBWs. PLoS One 9, e88392. https://doi.org/10.1371/journal.pone.0088392 (2014).
Acknowledgements
We thank Prof. Hidehiko Nakanishi (Division of Neonatal Intensive Care Medicine, Kitasato University) and Prof. Satoshi Kusuda (Department of Pediatrics, Kyorin University) for their support with data extraction and study design.
Institutions enrolled in the study of the Neonatal Research Network, Japan were as follows: Sapporo City General Hospital, Asahikawa Kosei General Hospital, Engaru-Kosei General Hospital, Kushiro Red Cross Hospital, Obihiro-Kosei General Hospital, Tenshi Hospital, NTT Higashinihon Sapporo Hospital, Nikko Memorial Hospital, Nayoro City General Hospital, Sapporo Medical University, Asahikawa Medical University, Aomori Prefectural Central Hospital, Iwate Medical University, Iwate Prefectural Ofunato Hospital, Iwate Prefectural Kuji Hospital, Iwate Prefectural Ninohe Hospital, Sendai Red Cross Hospital, Akita Red Cross Hospital, Tsuruoka Municipal Shonai Hospital, Yamagata University, Yamagata Prefectural Central Hospital, Fukushima Medical University, Takeda General Hospital, Fukushima National Hospital, Tsukuba University, Tsuchiura Kyodo Hospital, Ibaraki Children’s Hospital, Dokkyo Medical University, Jichi Medical University, Ashikaga Red Cross Hospital, Gunma Children’s Medical Center, Kiryu Kosei General Hospital, Fuji Heavy Industries Health Insurance Society Ota Memorial Hospital, Gunma University, Saitama Children’s Medical Center, Nishisaitama-chuo National Hospital, Saitama Medical University Saitama Medical Center, Kawaguchi Municipal Medical Center, Jichi Medical University Saitama Medical Center, Asahi General Hospital, Chiba Kaihin Municipal Hospital, Kameda Medical Center, Tokyo Women’s Medical University Yachiyo Medical Center, Juntendo University Urayasu Hospital, Tokyo Metropolitan Children’s Medical Center, Tokyo Women’s Medical University, Aiiku Hospital, Nihon University Itabashi Hospital, National Center for Global Health and Medicine, Tokyo Medical University, Teikyo University, Showa University, Japan Red Cross Medical Center, National Center for Child Health and Development, Tokyo Metropolitan Otsuka Hospital, Toho University, Tokyo Metropolitan Bokuto Hospital, Tokyo Jikei Medical University, Tokyo Medical and Dental University, Saint Luku’s International Hospital, Juntendo University, Sanikukai Hospital, Katsushika Red Cross Hospital, Yokohama Rosai Hospital, Yokohama City University Medical Center, St. Marianna University School of Medicine Hospital, Kanagawa Children’s Medical Center, Tokai University, Kitazato University, Odawara Municipal Hospital, Nippon Medical School Musashi Kosugi Hospital, Saiseikai Yokohamashi Tobu Hospital, National Hospital Organization Yokohama Medical Center, Yamanashi Prefectural Central Hospital, Nagano Children’s Hospital, Shinshu University, Iida Municipal Hospital, National Hospital Organization Shinshu Ueda Medical Center, Saku General Hospital, Niigata University, Niigata Prefectural Central Hospital, Niigata Municipal Hospital, Nagaoka Red Cross Hospital, Koseiren Takaoka Hospital, Toyama Prefectural Central Hospital, Toyama University, Ishikawa Medical Center for Maternal and Child Health, Kanazawa Medical University, Kanazawa Medical Center, Fukui Prefectural Hospital, Fukui University, Gifu Prefectural General Medical Center, National Hospital Organization Nagara Medical Center, Takayama Red Cross Hospital, Seirei Hamamatsu Hospital, Shizuoka Saiseikai Hospital, Shizuoka Children’s Hospital, Hamamatsu Medical University, Numazu Municipal Hospital, Yaizu City Hospital, Fujieda Municipal General Hospital, Nagoya Red Cross Daini Hospital, Nagoya University, Nagoya Red Cross Daiichi Hospital, Toyohashi Municipal Hospital, Nagoya City West Medical Center, Anjo kosei Hospital, Tosei General Hospital, Komaki Municipal Hospital, TOYOTA Memorial Hospital, Okazaki Municipal Hospital, Konan Kosei Hospital, National Mie Central Medical Center, Ise Red Cross Hospital, Yokkaichi Municipal Hospital, Otsu Red Cross Hospital, Shiga University of Medical Science Hospital, Nagahama Red Cross Hospital, Uji Tokushukai Hospital, The Japan Baptist Hospital, Kyoto University, Kyoto Red Cross Daiichi Hospital, National Maizuru Medical Center, Fukuchiyama City Hospital, Kyoto Prefectural University of Medicine Hospital, Kyoto City Hospital, Mitsubishi Kyoto Hospital, Yodogawa Christian Hospital, Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka University, Takatsuki General Hospital, Kansai Medical University, Osaka City General Hospital, Osaka City Sumiyoshi Hospital, Aizenbashi Hospital, Toyonaka Municipal Hospital, National Cerebral and Cardiovascular Center, Kitano Hospital, Saiseikai Suita Hospital, Chifune Hospital, Bellland General Hospital, Rinku General Medical Center, Osaka Red Cross Hospital, Yao Municipal Hospital, Osaka General Medical Center, Osaka City University, Hyogo Prefectural Kobe Children’s Hospital, Kobe University, Kakogawa West City Hospital, Saiseikai Hyogoken Hospital, Kobe City Medical Center General Hospital, Hyogo College of Medicine Hospital, Himeji Red Cross Hospital, Toyooka Public Hospital, Hyogo Prefectural Awaji Medical Center, Nara Medical University, Wakayama Medical University, Tottori Prefectural Central Hospital, Tottori University, Shimane Prefectural Central Hospital, Matsue Red Cross Hospital, Kurashiki Central Hospital, Tsuyama Central Hospital, Kawasaki Medical School Hospital, National Hospital Organization Okayama Medical Center, Okayama Red Cross Hospital, Hiroshima City Hiroshima Citizens Hospital, Hiroshima Prefectural Hospital, Hiroshima University, Tsuchiya General Hospital, National Hospital Organization Kure Medical Center, Yamaguchi University, Yamaguchi Grand Medical Center, Tokushima University, Tokushima Municipal Hospital, Kagawa University, National Hospital Organization Kagawa Children’s Hospital, Matsuyama Red Cross Hospital, Ehime Prefectural Central Hospital, Kochi Health Science Center, St. Mary’s Hospital, National Kyushu Medical Center, Kurume University, Kitakyushu Municipal Medical Center, University of Occupational and Environmental Health, Fukuoka University, Kyushu University, Iizuka Hospital, National Hospital Organization Kokura Medical Center, National Hospital Organization Saga Hospital, National Hospital Organization Nagasaki Medical Center, Kumamoto City Hospital, Kumamoto University, Oita Prefectural Hospital, Almeida Memorial Hospital, Nakatsu Municipal Hospital, Miyazaki University, National Hospital Organization Miyakonojo Medical Center, Kagoshima City Hospital, Imakiire General Hospital, Okinawa Prefectural Nanbu Medical Center & Children’s Medical Center, Okinawa Prefectural Chubu Hospital, Naha City Hospital, Okinawa Red Cross Hospital.
Author information
Authors and Affiliations
Contributions
S.S. and K.F. contributed to the conception and design of the study; S.S. contributed to the acquisition of the data; S.S. and K.F. performed statistical analyses, interpreted the data, and drafted the manuscript; Y.K., T.K., R.N., S.A., M.A., and K.N. critically reviewed the manuscript. All authors read and approved the final version of this manuscript, for publication.
Corresponding author
Ethics declarations
Competing interests
This work was partially supported by JSPS KAKENHI Grant Number 20K08229 and 20H00102 (Japan). The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suga, S., Kyono, Y., Kido, T. et al. Long use of continuous positive airway pressure protects against the development of treatment-requiring retinopathy of prematurity. Sci Rep 12, 7799 (2022). https://doi.org/10.1038/s41598-022-11509-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11509-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.