Abstract
The Hippo pathway is conserved and plays important roles in organ size control. The core components of the Hippo pathway are two kinases Hippo (Hpo), Warts (Wts), and a transcription-co-activator Yorkie (Yki). Yki activity is regulated by phosphorylation, which affects its nuclear localization and stability. To determine the role of the Hippo pathway in stem cells, we examine follicle stem cells (FSCs) in the Drosophila ovary. Yki is detected in the nucleus of FSCs. Knockdown of yki in the follicle cell lineage leads to a disruption of the follicular epithelium. Mitotic clones of FSCs mutant for hpo or wts are maintained in the niche and tend to replace the other FSCs, and FSCs mutant for yki are rapidly lost, demonstrating that the Hippo pathway is both required and sufficient for FSC maintenance. Using genetic interaction analyses, we demonstrate that the Hedgehog pathway acts upstream of the Hippo pathway in regulating FSC maintenance. The nuclear localization of Yki is enhanced when the Hedgehog signaling is activated. Furthermore, a constitutively active but not a wild-type Yki promotes FSC maintenance as activation of the Hedgehog signaling does, suggesting that the Hedgehog pathway regulates Yki through a post-translational mechanism in maintaining FSCs.
Similar content being viewed by others
Introduction
Stem cells undergo self-renewal to produce daughter cells with identical properties of the mother cell and daughter cells that differentiate into different types of cells. During developmental and adult stages, stem cells play critical roles to sustain tissue growth and homeostasis by expanding cell numbers and replacing aged or injured cells. Thus, it is important to understand molecular mechanisms underlying stem cell maintenance. Extrinsic signals and adherence molecules provided by the surrounding microenvironment, known as the niche, are essential for stem cells maintenance1, 2. Intrinsic signaling networks are also necessary for regulating stem cell fate and differentiation3.
To study signaling pathways regulating stem cells, the Drosophila ovary is an excellent model given its simple structure and convenient genetic tools4. The Drosophila ovary is comprised by fifteen to twenty tubular structures called ovarioles5. The most anterior part of the ovariole is a structure called the germarium, which contains germline stem cells (GSCs) and follicle stem cells (FSCs)6. Two to three GSCs are located at the anterior tip of the germarium, where they continuously divide to produce germline cysts7. Once the germline cyst passes through the junction between regions 2a and 2b of the germarium, it is wrapped by the follicular epithelium derived from FSCs. There are two FSCs in each germarium8. FSCs produce follicle cell precursors which differentiate into three types of cells: polar cells, stalk cells, and main-body follicle cells. Polar cells are specified early during oogenesis and play important roles in axis determination of the oocyte. Stalks cells separate adjacent egg chambers. Main-body follicle cells encapsulate the germline cyst to form the egg chamber. From cell division of GSCs to a mature egg, the process of oogenesis can be divided into fourteen stages based on the size and cell cycle status of germline and follicle cells9. Main-body follicle cells undergo eight to nine rounds of cell divisions to maintain a mono-layered epithelium surrounding the egg chamber before they enter endocycle at stage 6. These follicle cells are important for yolk production and eggshell formation in the following steps of oogenesis10, 11.
The FSC in the germarium is a well-characterized epithelial stem cell12. Many adhesion molecules and signaling pathways have been shown to contribute to FSC maintenance. FSCs mutant for shotgun (shg), which encodes Drosophila E-Cadherin (DE-Cad), are rapidly lost from the germarium, demonstrating that the adherence to nearby cells is required for FSC maintenance13. Escort cells immediately adjacent to FSCs serve as dynamic niche cells by providing ligands of Hedgehog pathway14. In addition to escort cells, cap cells in the anterior region of the germarium also secrete BMP and Wingless ligands to maintain FSCs15, 16. Interestingly, a recent study suggests that FSC maintenance is controlled by coordinative activity of both Hedgehog and JAK-STAT pathways17. Hedgehog (Hh) is released from the terminal filament and cap cells18. For the Hedgehog pathway, a transmembrane protein Patched (Ptc) constitutively represses the signaling activity by inhibiting Smoothened (Smo), a seven-transmembrane protein belonging to the G protein-coupled receptor (GPCR) superfamily19. The interaction of Hh and Ptc results in Smo activation, which acts through intracellular signaling complexes to convert the transcription factor Cubitus interruptus (Ci) from a truncated repressor to a full-length activator20. FSCs mutant for ptc are strongly maintained in the niche; and FSCs mutant for smo or ci are rapidly lost21. The JAK-STAT ligand Unpaired (Upd) is secreted from polar cells. FSCs mutant for genes encoding for Drosophila Janus Kinase Hopscotch (Hop) or Stat Stat92E, are lost from the niche17. Together, these data demonstrate that multiple signaling pathways contribute to FSC maintenance. Recently, a key component of the Hippo pathway Yorkie (Yki) has also been suggested to regulate FSC maintenance downstream of the co-activator of Notch signaling Mastermind (Mam) and the Hedgehog pathway22. However, the molecular mechanism underlying the interaction between the Hippo and Hedgehog pathways remains unclear.
The Hippo pathway is conserved from Drosophila to human and important for organ size control. Its core components include Hippo (Hpo), Warts (Wts), Salvador (Sav), Mob as a tumor suppressor (Mats), and Yorkie (Yki). Hpo is an Ste20-like kinase that forms a complex with the adaptor protein Sav and phosphorylates Wts, a nuclear Dbf2-related (NDR) family kinase23,24,25,26. In association with the adapter protein Mats27, Wts phosphorylates Yki, a transcriptional coactivator28. Phosphorylated Yki is retained in the cytosol and degraded29, 30. When Yki is not phosphorylated, it is imported to the nucleus and interacts with transcription factors such as Scalloped (Sd) to induce target genes such as cyclin E, Myc, Diap, expanded (ex), or bantam to promote cell proliferation and survival31. Interestingly, gene expression profiles do not overlap much among studies with different tissues or cells in Drosophila and mammals32,33,34,35,36, suggesting that Yki and its homologues YAP/TAZ in mammals may control gene expression in a tissue-specific manner. Thus, we should be cautious to take the expression of Yki target genes as indication of the transcriptional activity of Yki in specific cell types or tissues. Since the Hippo pathway is critical in growth control, various signals act upstream of the Hippo pathway, including cell polarity and cell junctions, cell adhesion, cell-cell contact and mechanical cues, cytoskeletons, G protein-coupled receptor signaling and cell metabolic status37. Moreover, the Hippo pathway interacts with Wnt, BMP, Notch, and Hedgehog pathways in various tissues and organisms38. Thus, it is important to understand how the Hippo pathway integrates such complicated signaling networks to modulate cellular functions.
We have previously discovered roles of the Hippo pathway in cell fate determination and migration of the follicle cell lineage39, 40. In this study, we showed that the Hippo pathway is both required and sufficient for FSC maintenance. We further used genetic interactions to demonstrate that the Hippo pathway acts downstream of the Hedgehog signaling. While ptc mutant FSCs were maintained, ptc and yki double-mutant FSCs were lost quickly as that of yki mutant FSCs. Reduction of wts partially rescued the maintenance defect of smo mutant FSCs. Both the level and nuclear distribution of Yki were increased in ptc mutant clones. Importantly, over-expression of a constitutively active but not wild-type yki promoted FSC maintenance as over-activation of the Hedgehog pathway did, suggesting that the Hedgehog pathway regulates Yki activity through a post-translational mechanism. Together, our data demonstrate that the Hippo pathway acts downstream of the Hedgehog signaling in regulating FSC maintenance.
Results
Yki is detected in the follicle cell lineage
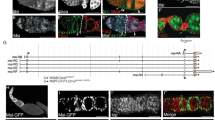
To investigate roles of the Hippo pathway during oogenesis, we first examined the expression pattern of Yki by generating a polyclonal antibody against Yki (anti-Yki). Yki was detected in the follicle cell lineage but not germline cells (Fig. 1A). To distinguish different follicle cell types, we stained ovaries with anti-Fasciclin III (FasIII), which labels follicle cell precursors in the germarium and polar cells in the developing egg chamber (Fig. 1A)41. FSCs were identified by their localization between 2a and 2b regions of the germarium and a low level of FasIII (Fig. 1A,B). The immunofluorescent staining pattern of our anti-Yki is consistent with that of the anti-Yki from Dr. Kenneth Irvine’s laboratory29, 39. Since Yki is a transcriptional co-activator, its nuclear localization is critical for its function29, 42. In the follicle cell lineage, Yki was detected in the nucleus of FSCs, follicle cell precursors, and follicle cells prior to stage 5 when they were in mitotic cell cycle (Fig. 1A–C). Importantly, Yki was not detected in the nucleus of polar cells, which withdrew from cell cycle after stage 2 (Fig. 1A)39. Yki was not detected in the nucleus of follicle cells after stage 5 when they entered endocycle (Fig. 1D). No immunofluorescent signal was detected in yki mutant clones, demonstrating the specificity of our anti-Yki (Fig. 1E). Yki activity has been examined previously by using reporters such as Diap-LacZ and ex-LacZ in the intestine and discs, where cells continuously undergo proliferation28, 43,44,45. Unexpectedly, both Diap-LacZ and ex-LacZ were expressed in polar cells and follicle cells withdrawn from mitosis at stage 7 (Supplementary Fig. S1). These cells expressing ex-LacZ and diap-LacZ did not show nuclear localization of Yki. While functional analyses have demonstrated that Yki functions to inhibit polar cell fate and promote mitosis in follicle cells39, 46, it is likely that Yki does not target ex or Diap in the follicle cell lineage. To further examine whether the Hippo pathway regulates the expression of Diap-LacZ and ex-LacZ, we generated yki or hpo mutant clones in the background of Diap-LacZ as well as wts mutant clones in the background of ex-LacZ. The intensities of β-GAL in yki mutant follicle cell precursors and follicle cells at stage 5 were not significantly reduced comparing with the neighboring GFP-positive control cells (Fig. S2A,B). Consistently with previous findings46, 47, Diap-LacZ was strongly increased in hpo mutant posterior follicle cells and ex-LacZ was strongly increased in wts mutant posterior follicle cells after stage 6 (Fig. S2D,F). However, the intensity of β-GAL in hpo or wts mutant follicle cell precursors was similar to that of the neighboring GFP-positive control cells (Fig. S2C,E). This result support that the Hippo pathway does not regulate ex or Diap in the follicle cell lineage during early oogenesis. Therefore, we used the level and distribution of Yki immunofluorescent staining instead of those Yki activity reporters in our following experiments.
The level and distribution of Yki in the follicle cell lineage. Ovaries from w 1118 were stained with anti-Yki, anti-FasIII or anti-GFP. Nuclear DNA was stained with DAPI. Samples were oriented as anterior to the left. (A) Immunostaining pattern of Yki in an ovariole with the germarium and egg chambers at stages 2, 3, 5 and 8. Yki was detected in the follicle lineage. The level of Yki was high in FSCs and follicle cell precursors, and decreased gradually. In polar cells (white arrowheads) and follicle cells at stage 8, Yki was excluded from the nucleus. (B) A high level of Yki was observed in FSCs (white arrowheads). Yki was detected at similar level in both the cytoplasm and nucleus. (C) A high level of Yki was detected in main-body follicle cells prior to stage 5. The levels of Yki were similar in the cytoplasm and nucleus. (D) At stage 8, the Yki level was decreased and excluded from the nucleus. (E) No Yki signal was detected in GFP-negative yki mutant clones (yellow arrowheads) six days after clone induction. Scale bar length is 10 μm.
Yki is required for maintaining the follicle cell lineage
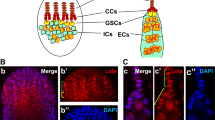
Since Yki was localized in the nuclei of FSCs, we further examined the function of Yki by using UAS-GAL4 system to knock down or over-express yki. By crossing with a UAS-gfp line, we showed that traffic jam (tj)-GAL4 was expressed in most cells of the follicle cell lineage including FSCs and c587-GAL4 was expressed abundantly in FSCs and escort cells (Fig. 2A,B). Knockdown of yki with either tj-GAL4 or c587-GAL4 severely disrupted the follicular epithelium (Fig. 2C,D), which might be resulted from the loss of FSCs. Most remaining follicle cells were positive for FasIII (Fig. 2C,D). Although FasIII is detected in both follicle cell precursors and polar cells, our previous study clearly demonstrates that loss of Yki function promotes polar cells differentiation39. Thus it is likely that these FasIII-positive cells resulted from yki knockdown are polar cells (Fig. 2C,D). Nuclear localization and activity of Yki is regulated by Ser/Thr phosphorylation. Ser residues at positions 111, 168, and 250 of Yki are phosphorylated by Wts and the phosphorylation prevents its nuclear localization. Since the Ser 111, 168, 250 to Ala mutations of Yki promote its nuclear import, we over-expressed yki-S111A, S168A, S250A (yki-3SA) instead of the wild-type yki. Previous studies have shown that Castor (Cas) is detected in FSCs and follicle cell precursors48 (Fig. 2E). Over-expression of yki-3SA with c587-GAL4 increased Cas-positive cells anterior to the region 2a/2b junction (Fig. 2F), suggesting that constitutive activation of Yki might result in ectopic FSCs. Together, our data suggested that Yki plays an important role in the follicle cell lineage, possibly in FSCs.
Yki is crucial for the follicle cell lineage. The flies were grown at 29 °C for six days. Ovaries were dissected and stained with anti-FasIII, anti-GFP (A,B), anti-Cas (E,F) and DAPI. Samples were oriented as anterior to the left. (A) tj-GAL4 was used to drive gfp expression. GFP was detected in escort cells, FSCs (white arrowheads), follicle cell precursors and main-body follicle cells. (B) c587-GAL4 was used to drive gfp expression. GFP was detected in escort cells and FSCs (white arrowheads), but not in follicle precursors or follicle cells. (C,D) When yki-RNAi was driven by tj-GAL4 or c587-GAL4, the entire follicular epithelium was disrupted. Ectopic FasIII-positive cells were observed. (E) FSCs (white arrowheads) and follicle cell precursors were Cas-positive in the germarium of c587-GAL4. (F) yki-3SA was driven by c587-GAL4. In the germarium, ectopic Cas-positive cells anterior to the FSC were observed (yellow arrowheads). Scale bar length is 10 μm.
The Hippo pathway regulates follicle stem cell maintenance
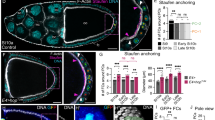
We next examined the maintenance of FSCs mutant for yki, hpo or wts. Mitotic clones generated by the Flipase/FRT system were identified by not expressing GFP (Fig. 3A–D). FRT42D and FRT82B clones were generated as controls. After clone induction, ovaries were dissected at different time points and the percentage of germaria containing mutant FSC clones was calculated (Fig. 3E,F). In FRT42D and FRT82B groups, most GFP-negative FSCs were maintained in the germarium thirteen days after clone induction (Fig. 3A,E, Fig. S3A). The percentage of germaria containing two GFP-negative FRT42D or FRT82B FSCs increased slowly after clone induction, suggesting that some GFP-positive FSCs have been replaced by their neighboring GFP-negative FSCs (Fig. 3F). yki mutant FSCs were rapidly lost and differentiated into FasIII-positive polar cells (Fig. 3B,E)39. Most hpo or wts mutant clones were maintained in the germarium (Fig. 3C–E). Importantly, more germaria contained two hpo or wts mutant FSCs than that of the FRT42D and FRT82B control groups thirteen days after clone induction, suggesting that hpo or wts mutant FSCs tend to stay in the niche and replace the neighboring GFP-positive control FSC (Fig. 3F). Consistently, sav mutant FSCs tended to replace the GFP-positive neighboring control FSC (Fig. S3B,E); sd mutant FSCs were rapidly lost from the germarium (Fig. S3D, F). These data are consistent with a previous study and demonstrate that core components of the Hippo pathway are important for regulating FSC maintenance22.
The Hippo pathway regulates FSC maintenance. Ovaries were dissected thirteen days after clone induction and stained with anti-GFP, anti-FasIII, and DAPI. Mitotic clones were GFP-negative. (A) A germarium with one GFP-negative (yellow arrowheads) and one GFP-positive (white arrowheads) FSCs in FRT42D control. (B) A germarium with two GFP-positive FSCs (white arrowheads). FasIII-positive yki mutant cells were observed in a stage 2 egg chamber (yellow arrows). (C) A germarium with two GFP-negative hpo mutant FSCs (yellow arrowheads). (D) A germarium with two GFP-negative wts mutant FSCs (yellow arrowheads). (E,F) Germaria were categorized based on the numbers of GFP-negative FSCs in a germarium three, six, and thirteen days after clone induction. Pearson’s chi-squared test was used for statistic analysis. n ≥ 180 for each genotype/time point. **p < 0.01. (E) The percentages of germaria with one or two GFP-negative FSCs were shown. The percentages of germaria with one or two FRT42D, FRT82B, hpo, or wts mutant FSCs were maintained over time. The percentages of germaria with one or two yki mutant FSCs were decreased drastically. (F) The percentages of germaria with two GFP-negative FSCs were shown. The percentages of germaria with two FRT42D or FRT82B GFP-negative FSCs were increased slowly. Few germarium with two yki mutant FSCs was observed six or thirteen days after clone induction. The percentage of germaria with two hpo or wts mutant FSCs were increased dramatically. Scale bar length is 10 μm (A–D).
Confirmation of the interaction between the Hippo and Hedgehog pathways
Similar to the Hippo pathway, it has been shown that the Hedgehog pathway is required and sufficient for FSC maintenance21, so we tested whether the Hippo and Hedgehog pathways may interact with each other in the follicle cell lineage. We have previously shown that mutation of wts did not affect the expression of ptc-lacZ, a reporter for the Hedgehog signaling activity39, suggesting that the Hippo pathway does not act upstream of the Hedgehog pathway. It has been demonstrated that the Hedgehog pathway acts upstream of the Hippo pathway in regulating FSC maintenance based on the expression of Diap-LacZ 22. Since the expression of Diap-LacZ does not reflect the Hippo pathway activity during early oogenesis (Fig. S2), we examined the level of Yki in patched (ptc) mutant cells. Ptc is a key negative regulator of the Hedgehog pathway. The Yki level was significantly increased in ptc mutant cells in follicle cell precursors (Fig. 4A). This result confirmed that the Hippo pathway acts downstream of the Hedgehog signaling in the follicle cell lineage.
Yki acts downstream of the Hedgehog pathway in promoting FSC maintenance. Ovaries were dissected six days (A) or thirteen days (B,C) after clone induction and stained with anti-GFP, anti-FasIII, anti-Yki (A,B), and DAPI. Mitotic clones were GFP-negative. (A) The intensity of Yki immunofluorescent staining was increased in ptc mutant follicle cells in the germarium. (B) A germarium with two GFP-negative ptc mutant FSCs (yellow arrowheads) were shown. (C) A germarium with two GFP-positive FSCs (white arrowheads). ptc, yki double mutant cells left the germarium and were FasIII-positive (yellow arrows). (D) The percentages of germaria with one or two ptc, yki double mutant FSCs were decreased drastically over time. (E) The percentages of germaria with two GFP-negative FRT42D control FSCs were increased slowly over time. No germarium with two ptc, yki double mutant FSCs was observed thirteen days after clone induction. The percentage of germaria with two ptc mutant FSCs were increased dramatically. Pearson’s chi-squared test was used for statistic analysis. n ≥ 180 for each genotype/time point. Scale bar length is 10 μm.
We further examined the genetic interaction between the Hippo and Hedgehog pathway demonstrated previously by Kalderon’s laboratory22. ptc mutation leads to over-activation of the Hedgehog pathway and strongly promotes FSC maintenance21. If the Hippo pathway acts downstream of the Hedgehog pathway, ptc yki-double mutant FSCs should be lost from the germarium as yki mutant FSCs. FRT42D clones were generated as controls. Consistent with previous results21, ptc mutant FSCs were maintained in the germarium and more germaria contained two ptc mutant FSCs than that of the FRT42D control thirteen days after clone induction (Fig. 4B,D and E), confirming that ptc mutant FSCs tend to replace the GFP-positive neighboring control FSCs. Similar to that of yki mutant FSCs, ptc yki-double mutant FSCs were rapidly lost (Fig. 4C–E), supporting our hypothesis that the Hippo pathway acts downstream of the Hedgehog pathway in regulating FSC maintenance.
We further tested whether reduction of wts might promote maintenance of FSCs mutant for smo, a positive regulator of the Hedgehog pathway. FRT40A clones were used as a control. Comparing with FRT40A control FSCs, smo mutant FSCs were rapidly lost (Fig. 5A,B and D). In a wts heterozygous background, the percentage of germaria containing smo mutant FSCs were increased at thirteen days after clone induction (Fig. 5C,D). However, the percentage of germaria containing two smo mutant FSCs were not rescued by reduction of wts (Fig. 5E). Thus, reduction of wts partially rescued the FSC maintenance defect of smo mutation. Our data provided further evidence that the Hippo pathway acts downstream of the Hedgehog pathway in regulating FSC maintenance.
Reduction of wts promotes maintenance of smo mutant FSCs. Ovaries were dissected thirteen days after clone induction and stained with anti-GFP, anti-FasIII, and DAPI. Mitotic clones were GFP-negative. (A) A germarium with one GFP-negative (yellow arrowheads) and one GFP-positive (white arrowheads) FRT40A control FSC were shown. (B) A germarium with two GFP-positive FSCs (white arrowheads) were shown. smo mutant cells left the niche (yellow arrows). (C) A germarium with one smo mutant GFP-negative FSC (yellow arrowheads) and one GFP-positive FSC (white arrowheads) in a wts heterozygous background were shown. (D) The percentage of germaria with one or two smo mutant FSCs was decreased drastically. In a wts heterozygous mutant background, however, the percentage of germaria containing one or two smo mutant FSCs was increased. (E) The percentages of germaria with two GFP-negative FRT40A FSCs were increased slowly. Few germarium with two smo mutant FSCs was observed. In the wts heterozygous mutant background, some of germaria with two smo mutant FSCs were observed. Pearson’s chi-squared test was used for statistic analysis. n ≥ 180 for each genotype/time point. Scale bar length is 10 μm.
The Hedgehog pathway regulates the Hippo pathway through a post-transcriptional mechanism
It has been shown that the Hedgehog pathway transcriptionally promotes yki expression in the follicle cell lineage22. Since we have demonstrated that the Yki level is increased in ptc mutant cells, it is important to investigate whether the Hedgehog pathway regulates nuclear localization of Yki through a post-translational mechanism. FRT42D control and ptc mutant follicle cell clones were generated and the immunofluorescent staining intensities of Yki in the nucleus or in the whole cell were quantified (Fig. 6). The ratio of nuclear Yki immunofluorescent intensity to the total intensity was significantly higher in ptc mutant cells than that of the FRT42D control clones (Fig. 6C). This result suggested that the Hedgehog pathway might regulate nuclear localization of Yki in addition to the transcriptional regulation proposed by a previous study22.
The Hedgehog pathway regulates nuclear distribution of Yki. Ovaries were dissected six days after clone induction and stained with anti-GFP, anti-Yki, and DAPI. Stage 3 to 4 egg chambers were selected. Mitotic clones were GFP-negative. (A) In FRT42D control clones, Yki was detected in both nucleus and cytoplasm. (B) In ptc mutant, the levels of Yki in the whole cell as well as in the nucleus were increased. (C) Quantification of the intensity of Yki immunofluorescent staining and the ratio of the intensity in the nucleus to that of the whole cell. The ratio was significantly higher in ptc mutant cells than that of FRT42D control cells. The bar graph is shown as Mean ± SEM. Student’s t-test is used for statistic analysis. n ≥ 26 for each group. **p < 0.05. Scale bar length is 10 μm.
If the Hedgehog pathway exclusively regulates FSC maintenance by increasing transcription of yki 22, increase of wild-type yki expression alone should be sufficient to promote FSC maintenance as ptc mutation does. We used the FLP-out technique with act-GAL4 to generate clones over-expressing yki or yki-3SA and examine FSC maintenance. Both UAS-yki and UAS-yki-3SA were inserted into the same locus through site-specific integration to minimize the variation of transgene expression49. FLP-out clones expressing gfp were generated as a control. In the control group (OE gfp), most GFP-positive FSCs were maintained thirteen days after clone induction (Fig. 7A,D and E). Over-expression of yki showed similar FSC maintenance as the control (Fig. 7B,D and E), suggesting that increase of yki expression alone does not promote FSC maintenance. On the other hand, over-expression of yki-3SA led to a higher percentage of germaria containing one or two GFP-positive FSCs than that of the control or yki over-expression (Fig. 7C–E). Together with data in Fig. 6, our results suggested that increase of Yki nuclear localization, but not merely yki expression, promotes FSC maintenance. While activation of the Hedgehog pathway strongly promotes FSC maintenance, it is likely that the Hedgehog pathway regulates Yki through a post-translational mechanism in addition to the previously proposed transcriptional regulation22.
Yki phosphorylation is key for regulating FSC maintenance. Ovaries were dissected thirteen days after clone induction and stained with anti-GFP, anti-FasIII, and DAPI. FLP-out clones were GFP-positive. (A) A germarium with one GFP-negative FSC (white arrowheads) and one GFP-positive FSC (yellow arrowheads) in the control group were shown. (B) A germarium with one GFP-negative (white arrowheads) FSC and one GFP-positive FSC over-expressing yki (yellow arrowheads) were shown. (C) A germarium with two GFP-positive FSCs over-expressing yki-3SA (yellow arrowheads) were shown. Scale bar length is 10 μm. (D,E) The percentages of germaria with one or two GFP-positive FSCs (D), or two GFP-positive FSCs (E) over-expressing gfp (control), yki, or yki-3SA at different time points were shown. The percentage of yki-3SA group with one or two GFP-positive FSCs was significantly higher than that of gfp control and yki groups thirteen days after clone induction. Pearson’s chi-squared test was used for statistic analysis. n ≥ 180 for each genotype/time point.
Discussion
In this study, we show that inhibition of Yki function results in loss of the FSC and disruption of the follicular epithelium. Over-activation of Yki by either expression of a constitutively active form of yki or mutation of genes encoding for negative regulators of Yki such as hpo, wts, or sav promotes FSC maintenance. These data suggest that proper activity of the Hippo pathway is critical for FSC maintenance. However, underlying mechanisms of the Hippo pathway in regulating FSC maintenance remains to be identified. We have previously shown that inactivation of Yki promotes polar cell fate and activation of Yki disrupts polar cell differentiation39. Thus, it is possible that inactivation of Yki leads to premature polar cell differentiation of FSCs, therefore results in FSC loss. In addition, the Hippo pathway has been shown to regulate multiple aspects of follicle cell functions through varieties of signaling pathways. In border cells, the Hippo pathway regulates polarized distribution of the actin cytoskeleton40, 50. In polar cells, the Hippo pathway regulates upd expression to control border cell induction and migration40. In addition, Wts regulates invasion of follicle cells into egg chambers in coordination with basolateral junctional components, such as Fasciclin 2 and Discs large 151. Thus, it remains possible that the Hippo pathway may control cytoskeleton, JAK/STAT signaling, or cellular junctions, which in turns affects FSC maintenance.
In addition to its function in the FSC, the Hippo pathway plays important roles in various types of stem cells in Drosophila, such as neural stem cells (NSCs) and intestinal stem cells (ISCs)52. At early larvae stage, NSCs may either stay in quiescence or proliferate in response to metabolic changes. Recent studies have shown that the Hippo pathway regulates the activity of several factors involved in asymmetric cell division, such as Mushroom body defect (Mud), Canoe/Afadin, and Bazooka, therefore control neural stem cell division and differentiation53, 54. In the intestine, the Hippo pathway is required in both niche cells and ISCs for regulating ISC proliferation, especially during injury-induced regeneration44, 45, 55. In either niche cells or ISCs, the Hippo pathway controls expression of upd to promote proliferation and survival of ISCs. These studies in other stem cells may provide hints to understand how the Hippo pathway controls FSC maintenance.
Here we show that the Hedgehog pathway acts upstream of the Hippo pathway in regulating FSC maintenance. Interestingly, although both the Hippo and Hedgehog pathways control cyst stem cell maintenance in the Drosophila testis, these two pathways do not interact with each other56. In the wing disc, mutation of ptc leads to overproliferation of surrounding cells through activation of Yki57, demonstrating that the Yki also acts downstream of the Hedgehog pathway in a non-autonomous manner. A recent study has shown that the Hedgehog controls Yki through Mastermind (Mam) at transcription level to regulate FSC maintenance22. They used Diap-LacZ as a reporter for Yki activity. However, the expression of Diap-LacZ does not reflect the activity of the Hippo pathway in the follicle cell lineage during early oogenesis (Supplementary Fig. S1A–C, Fig. S1A–D). Moreover, we did not observe changes of Yki level or nuclear localization in follicle cells either over-expression or mutation of mam as well as over-expression of a constitutive form of ci (Supplementary Fig. S4). It will be important to further investigate how Mam regulates FSC maintenance. The interaction between the Hippo and Hedgehog pathways has also been demonstrated in mammals. In cerebellar granule neuron precursors (CGNPs) and medullablastoma, activation of the Sonic Hedgehog pathway both induces Yap expression and promotes Yap nuclear localization58. Interestingly, activation of Yap increases the activity of the Sonic Hedgehog pathway in CGNPs and cortical progenitors, suggesting that these two pathways may form a positive feedback loop to promote expansion of neural progenitors and/or accelerate tumorigenesis. The Sonic Hedgehog pathway has also been shown to act downstream of the Hippo pathway in promoting proliferation and inhibiting neuronal differentiation in mouse cortical progenitors59. A previous study has demonstrated that the Hedgehog pathway regulates Yki at transcriptional level in the Drosophila ovary22. Since we observe an increase of Yki nuclear distribution in ptc mutant cells and over-expression of Yki alone does not promote FSC maintenance, we conclude that the Hedgehog pathway regulates Yki through a post-translational mechanism. While overall level of Yki is increased in ptc mutant clones (Figs 4A and 6B), we do not exclude the possibility that the Hedgehog pathway regulates Yki at transcriptional level. Thus, it is important to further investigate how these two pathways interact with each other in regulating stem cell properties.
Various upstream signals have been demonstrated to regulate the Hippo pathway, including the apical-basal polarity complex. Interestingly, we found that the level of aPKC is increased and the apical distribution of aPKC is disrupted in ptc mutant clones (Supplementary Fig. S5A–C), where the level and distribution of Yki are increased (Figs 4A and 6B). Therefore, it is possible that the Hedgehog pathway regulates the Hippo pathway through affecting apical-basal polarity. However, we did not observe significant change of the Yki level or nuclear distribution in aPKC knockdown follicle cells (Supplementary Fig. S5D). Since there is limitation in quantification of subtle change in Yki level or distribution based on immunofluorescence signals, it remains possible that the Hedgehog pathway regulates FSC maintenance through cell polarity. It is recently suggested that the apical-basal polarity of FSCs is unique by its broadly distributed adherens junctions and the lack of a mature apical domain. Mutation of two basolateral junction genes, lethal giant larvae (lgl) or discs large (dlg), promotes FSC maintenance60. While our data have shown interaction between the Hippo and Hedgehog pathways, as well as the Hedgehog pathway and cell polarity, it will be interesting to further examine how the Hippo pathway, the Hedgehog pathway, and cell polarity regulates stem cell maintenance in concert.
Methods
Fly stock
Fly lines used for over-expression and knockdown experiments: P{UAS-yki.V5.O}attP2 (Bloomington stock center BLM28819), P{UAS-yki.S111A.S168A.S250A.V5}attP2 (BLM28817), UAS-GFP, P{KK109756}VIE-260B (Vienna Drosophila Resource Center, V104523), P{GawB}NP1624/CyO, P{UAS-lacZ.UW14}UW14 (DGRC Kyoto Stock Center, DGRC 104055), P{GAL4}C587. tub-Gal80 ts /CyO (gift from Dr. G.J Liaw). Fly lines used for clonal analysis: hs-flp, ubiGFP FRT19A/(FM7c), hs-flp; ubiGFP FRT40A/CyO, hs-flp;FRT42D ubiGFP/CyO, hs-flp;;FRT82B ubiGFP, FRT19A, FRT40A, ey-flp;FRT42D, ey-flp;;FRT82B/TM3, Sb, FRT42D yki B5,w + /CyO, w −; FRT42D hpo 42,43,44,45,46,47,w + /CyO, FRT82B wts x1 /TM3,Sb, FRT42D ptc IIw /CyO, smo 3 FRT40A/CyO, FRT82B sav 3 /TM6b, sd ∆B FRT19A/FM7, w *; P{UAS-mam.A}2, y d2 w 1118 P{ey-FLP.N}2 P{GMR-lacZ.C(38.1)}TPN1; P{neoFRT}42D P{GT1}mam BG02477 /CyO y +, hs-flp;;P{GAL4-Act5C(FRT.CD2).P}/TM3, hs-flp;UAST-GFP act > CD2 > Gal4/(CyO). lacZ reporter and other lines include w 1118, y 1 sc * v 1; P{TRiP.HMS01320}attP2 (BLM 34332), w*; P{lacW}ex 697 /CyO; TM2/TM6B, Tb 1, y 1 w *; P{lacW}Diap1 j5C8 /TM3, Sb 1 (BLM 12093).
Antibodies and Reagents
The following antibodies were used at the indicated dilutions: mouse anti-Fasciclin III 1:200 [7G10, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-β-Galactosidase 1:200 (40-1A, DSHB), rabbit anti-GFP 1:1000 (Invitrogen, USA), rat anti-GFP (1:1000, Nacalai Tesque, Japan), Dylight-488 goat anti-rabbit IgG(H + L), Dylight-549 goat anti-mouse IgG(H + L) (Jackson ImmunoResearch Laboratories, USA), Alexa-546 Phalloidin 1:50 (Invitrogen). Yki rabbit polyclonal antibody was generated with full-length Yki29. Rabbit anti-Cas (1:5,000) was generous gifts from Dr. Chang and Dr. Jang48.
Overexpression and RNAi knockdown with UAS-GAL4 system
Flies were cultured at 18 °C before eclosion. Newly eclosed adult females were collected and incubated at 29 °C for six days before dissection.
Generation of mitotic clones
Mitotic clones were generated by using the FLP/FRT and Flip-out systems59, 60. Two days after eclosion, female flies were collected and heatshocked for four times in two days. They were heatshocked twice for 30 min each with a four-hour interval on the first day. On the second day, they were heatshocked once for 30 min and once for one hour with a four-hour interval. Flies with mutant clones were then incubated at 25 °C; flies with Flip-out clones were incubated at 29 °C.
Immunohistochemistry
Ovaries were dissected in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA) in PBS for 15 minutes. After fixation, ovaries were washed with PBT (1XPBS, and 0.2% Triton X-100) for 3 times, followed by incubation in blocking solution PBTB (1XPBS, 0.5% Triton X-100, 5% goat serum, 2.5 mg/ml BSA, and 0.05% Sodium azide) for 1 hour. Ovaries were then incubated with the primary antibodies overnight at 4 °C and followed by secondary antibody staining for 2 hours at room temperature. Ovaries were further stained with DAPI (1 μg/ml, Sigma) in PBT for 30 minutes prior mounting with mounting solution [85% glycerol, 1XPBS, 3% propyl gallate (Sigma), and ProLong® Gold Antifade reagent (Invitrogen, Carlsbad, CA, USA)].
Fluorescence microscopy
All the images were taken by Zeiss LSM700 confocal microscope (Carl Zeiss AG) and processed with Adobe Photoshop CS5 (Adobe). For quantification of fluorescence intensity of Yki immunofluorescence, cells with large area of DAPI staining were selected. The boundary of a cell and the nucleus were determined by FasIII immunostaining and DAPI staining, respectively. The read of average intensity in the field was measured by using ImageJ (NIH, Bethesda, MD).
References
Li, L. & Xie, T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol 21, 605–631, doi:10.1146/annurev.cellbio.21.012704.131525 (2005).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Yamashita, Y. M., Fuller, M. T. & Jones, D. L. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci 118, 665–672, doi:10.1242/jcs.01680 (2005).
Rubin, T. & Huynh, J. R. Mosaic Analysis in the Drosophila melanogaster Ovary. Methods Mol Biol 1328, 29–55, doi:10.1007/978-1-4939-2851-4_3 (2015).
Roth, S. Drosophila oogenesis: coordinating germ line and soma. Curr Biol 11, R779–781 (2001).
Kirilly, D. & Xie, T. The Drosophila ovary: an active stem cell community. Cell Res 17, 15–25 (2007).
Fuller, M. T. & Spradling, A. C. Male and female Drosophila germline stem cells: two versions of immortality. Science 316, 402–404 (2007).
Nystul, T. & Spradling, A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics 184, 503–515, doi:10.1534/genetics.109.109538 (2010).
Horne-Badovinac, S. & Bilder, D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn 232, 559–574 (2005).
Dobens, L. L. & Raftery, L. A. Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells. Dev Dyn 218, 80–93 (2000).
Deng, W. M. & Bownes, M. Patterning and morphogenesis of the follicle cell epithelium during Drosophila oogenesis. Int J Dev Biol 42, 541–552 (1998).
Klusza, S. & Deng, W. M. At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. Bioessays 33, 124–134, doi:10.1002/bies.201000089 (2011).
Song, X. & Xie, T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA 99, 14813–14818 (2002).
Sahai-Hernandez, P. & Nystul, T. G. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development 140, 4490–4498, doi:10.1242/dev.098558 (2013).
Kirilly, D., Spana, E. P., Perrimon, N., Padgett, R. W. & Xie, T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell 9, 651–662 (2005).
Song, X. & Xie, T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development 130, 3259–3268 (2003).
Vied, C., Reilein, A., Field, N. S. & Kalderon, D. Regulation of stem cells by intersecting gradients of long-range niche signals. Dev Cell 23, 836–848, doi:10.1016/j.devcel.2012.09.010 (2012).
Zhang, Y. & Kalderon, D. Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development 127, 2165–2176 (2000).
Jiang, J. & Hui, C. C. Hedgehog signaling in development and cancer. Dev Cell 15, 801–812, doi:10.1016/j.devcel.2008.11.010 (2008).
Huangfu, D. & Anderson, K. V. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3–14, doi:10.1242/dev.02169 (2006).
Zhang, Y. & Kalderon, D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 (2001).
Huang, J. & Kalderon, D. Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J Cell Biol 205, 325–338, doi:10.1083/jcb.201309141 (2014).
Edgar, B. A. From cell structure to transcription: Hippo forges a new path. Cell 124, 267–273 (2006).
Hariharan, I. K. & Bilder, D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet 40, 335–361 (2006).
Harvey, K. & Tapon, N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer 7, 182–191 (2007).
Pan, D. Hippo signaling in organ size control. Genes Dev 21, 886–897 (2007).
Lai, E. C., Roegiers, F., Qin, X., Jan, Y. N. & Rubin, G. M. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development 132, 2319–2332 (2005).
Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421–434 (2005).
Oh, H. & Irvine, K. D. In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081–1088 (2008).
Ren, F., Zhang, L. & Jiang, J. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol 337, 303–312, doi:10.1016/j.ydbio.2009.10.046 (2010).
Wu, S., Liu, Y., Zheng, Y., Dong, J. & Pan, D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 14, 388–398 (2008).
Oh, H. et al. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep 3, 309–318, doi:10.1016/j.celrep.2013.01.008 (2013).
Nagaraj, R. et al. Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes Dev 26, 2027–2037, doi:10.1101/gad.183061.111 (2012).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22, 1962–1971, doi:10.1101/gad.1664408 (2008).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17, 1218–1227, doi:10.1038/ncb3216 (2015).
Lian, I. et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 24, 1106–1118, doi:10.1101/gad.1903310 (2010).
Meng, Z., Moroishi, T. & Guan, K. L. Mechanisms of Hippo pathway regulation. Genes Dev 30, 1–17, doi:10.1101/gad.274027.115 (2016).
Yu, F. X. & Guan, K. L. The Hippo pathway: regulators and regulations. Genes Dev 27, 355–371, doi:10.1101/gad.210773.112 (2013).
Chen, H. J. et al. The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev Biol 357, 370–379, doi:10.1016/j.ydbio.2011.07.003 (2011).
Lin, T. H., Yeh, T. H., Wang, T. W. & Yu, J. Y. The Hippo pathway controls border cell migration through distinct mechanisms in outer border cells and polar cells of the Drosophila ovary. Genetics 198, 1087–1099, doi:10.1534/genetics.114.167346 (2014).
Ruohola, H. et al. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66, 433–449, doi:0092-8674(81)90008-8 [pii] (1991).
Oh, H. & Irvine, K. D. In vivo analysis of Yorkie phosphorylation sites. Oncogene 28, 1916–1927, doi:10.1038/onc.2009.43 (2009).
Karpowicz, P., Perez, J. & Perrimon, N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137, 4135–4145, doi:10.1242/dev.060483 (2010).
Ren, F. et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA 107, 21064–21069, doi:10.1073/pnas.1012759107 (2010).
Shaw, R. L. et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137, 4147–4158, doi:10.1242/dev.052506 (2010).
Yu, J., Poulton, J., Huang, Y. C. & Deng, W. M. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE 3, e1761 (2008).
Polesello, C. & Tapon, N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol 17, 1864–1870 (2007).
Chang, Y. C., Jang, A. C., Lin, C. H. & Montell, D. J. Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc Natl Acad Sci USA 110, E1734–1742, doi:10.1073/pnas.1300725110 (2013).
Fish, M. P., Groth, A. C., Calos, M. P. & Nusse, R. Creating transgenic Drosophila by microinjecting the site-specific phiC31 integrase mRNA and a transgene-containing donor plasmid. Nat Protoc 2, 2325–2331, doi:10.1038/nprot.2007.328 (2007).
Lucas, E. P. et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol 201, 875–885, doi:10.1083/jcb.201210073 (2013).
Zhao, M., Szafranski, P., Hall, C. A. & Goode, S. Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics 178, 1947–1971 (2008).
Mo, J. S., Park, H. W. & Guan, K. L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15, 642–656, doi:10.15252/embr.201438638 (2014).
Keder, A. et al. The hippo pathway core cassette regulates asymmetric cell division. Curr Biol 25, 2739–2750, doi:10.1016/j.cub.2015.08.064 (2015).
Ding, R., Weynans, K., Bossing, T., Barros, C. S. & Berger, C. The Hippo signalling pathway maintains quiescence in Drosophila neural stem cells. Nat Commun 7, 10510, doi:10.1038/ncomms10510 (2016).
Staley, B. K. & Irvine, K. D. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol 20, 1580–1587, doi:10.1016/j.cub.2010.07.041 (2010).
Amoyel, M., Simons, B. D. & Bach, E. A. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J 33, 2295–2313, doi:10.15252/embj.201387500 (2014).
Kagey, J. D., Brown, J. A. & Moberg, K. H. Regulation of Yorkie activity in Drosophila imaginal discs by the Hedgehog receptor gene patched. Mech Dev 129, 339–349, doi:10.1016/j.mod.2012.05.007 (2012).
Fernandez, L. A. et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev 23, 2729–2741, doi:10.1101/gad.1824509 (2009).
Lin, Y. T. et al. YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Exp Cell Res 318, 1877–1888, doi:10.1016/j.yexcr.2012.05.005 (2012).
Kronen, M. R., Schoenfelder, K. P., Klein, A. M. & Nystul, T. G. Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS One 9, e101085, doi:10.1371/journal.pone.0101085 (2014).
Acknowledgements
We thank G.-J. Liaw, H.-J. Hsu for discussion and critical reading of the manuscript; Y.-C. Tsai, H. Ruohola-Baker, Y.H. Sun, D. Pan, W.-M. Deng, J. Jiang, A. C.-C. Jang, the Fly Core in Taiwan, the Bloomington Stock Center, the Vienna Drosophila RNAi Center, and the Drosophila Genomics Resource Center for fly stocks; Y.-C. Chang, A. C.-C. Jang, and the Developmental Studies Hybridoma Bank for antibodies. This research is funded by the National Science Council Grants 101-2311-B-010-007-MY3, 104-2311-B-010-004, 105-2321-B-010-009 and Brain Research Center, National Yang-Ming University.
Author information
Authors and Affiliations
Contributions
Ta-Hsing Hsu designed and performed the experiments, analyzed the data, and wrote the manuscript. Chia-Yu Yang performed the experiments, analyzed the data, and wrote the manuscript. Tsung-Han Yeh designed and performed the experiments. Yi-Chia Huang designed and performed the experiments. Tsu-Wei Wang designed the experiments, analyzed the data, and wrote the manuscript. Jenn-Yah Yu designed and performed the experiments, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsu, TH., Yang, CY., Yeh, TH. et al. The Hippo pathway acts downstream of the Hedgehog signaling to regulate follicle stem cell maintenance in the Drosophila ovary. Sci Rep 7, 4480 (2017). https://doi.org/10.1038/s41598-017-04052-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04052-6
This article is cited by
-
HP1a-mediated heterochromatin formation inhibits high dietary sugar-induced tumor progression
Cell Death & Disease (2021)
-
Combined inhibition of JAK2-STAT3 and SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor growth, and metastasis
Oncogene (2020)
-
Hedgehog pathway inhibition causes primary follicle atresia and decreases female germline stem cell proliferation capacity or stemness
Stem Cell Research & Therapy (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.