Abstract
Starch phosphorylation occurs naturally during starch metabolism in the plant and is catalysed by glucan water dikinases (GWD1) and phosphoglucan water dikinase/glucan water dikinase 3 (PWD/GWD3). We generated six stable individual transgenic lines by over-expressing the potato GWD1 in rice. Transgenic rice grain starch had 9-fold higher 6-phospho (6-P) monoesters and double amounts of 3-phospho (3-P) monoesters, respectively, compared to control grain. The shape and topography of the transgenic starch granules were moderately altered including surface pores and less well defined edges. The gelatinization temperatures of both rice flour and extracted starch were significantly lower than those of the control and hence negatively correlated with the starch phosphate content. The 6-P content was positively correlated with amylose content and relatively long amylopectin chains with DP25-36, and the 3-P content was positively correlated with short chains of DP6-12. The starch pasting temperature, peak viscosity and the breakdown were lower but the setback was higher for transgenic rice flour. The 6-P content was negatively correlated with texture adhesiveness but positively correlated with the cohesiveness of rice flour gels. Our data demonstrate a way forward to employ a starch bioengineering approach for clean modification of starch, opening up completely new applications for rice starch.
Similar content being viewed by others
Introduction
Starch is the major carbohydrate of cereal grain and a primary dietary component of human energy intake. Grain starch also serves the major source for food and non-food applications such as in thickening, gelling, sizing, binding and adhesion. Owing to its non-optimal physicochemical properties, raw starch functionality does not meet industrial processing and product demands and therefore expensive and polluting post-harvest chemical modifications, e.g. phosphorylation producing so called “starch phosphate” to inhibit retrogradation, are frequently required1. Clean protocols for starch modification is therefore desired and starch bioengineering directly in the plant is an attractive approach2.
Phosphorylation is the only known in vivo covalent modification of starch. In the early 20th century, the presence of small amounts (0.2–0.4% w/w) of monoesterified phosphate groups was detected in potato (Solanum tuberosum) tuber starch3. Subsequently, it has been verified that phosphorylation takes place in virtually all plant species4, 5. In potato tuber starch, a majority of the phosphate monoesters (70–80%) are bound at the C-6 position of the glucosyl unit, while C-3 phosphorylation makes up 20–30%6. Two types of glucan water dikinaseshave been demonstrated to catalyse starch phosphorylation: α-Glucan, water dikinase 1 (GWD1; formerly designated as R1 or SEX1) and α-Glucan, water dikinase 3/phosphoglucan, water dikinase (PWD/GWD3). GWD1 specifically phosphorylates starch at the C-6 position, catalyzing the transfer of the β-phosphate from ATP to a glucosyl residue7 and then PWD recognizes the C-6 pre-phosphorylated glucan and subsequently phosphorylates C-3 hydroxyls8, 9. Phosphorylation by GWD1 disrupts the crystalline structure of the starch surface and increases the hydrolytic action of plastidial β-amylases10, 11. RNA interference-mediated down-regulation of GWD1 in the wheat endosperm resulted in decreased starch-bound phosphate, an increase in grain size and plant biomass but unaltered starch content12. Starch phosphorylation stimulates starch degradation in Arabidopsis mutant plants in which the activity of GWD1 or PWD/GWD3 is decreased. As a result, these plants exhibit significantly increased leaf starch contents with the phenotype of the GWD1-deficient plants being more severe8, 9, 13. However, the GWD1 enzyme level remains largely constant throughout the diurnal cycle and also starch biosynthesis seems to be affected by starch phosphorylation as demonstrated by both metabolic and structural effects in Arabidopsis 14 and barley15. Expression of the potato GWD1 in barley also affects grain germination in barley16. In support for a role of starch phosphorylation in both biosynthesis and degradation, GWD1 catalyzed phosphorylation of crystalline starch induces physical repulsion between starch segments5 thereby solubilising crystalline structures17 providing accessibility for biosynthetic or hydrolytic enzymes. Hence, GWD1 and PWD/GWD3 play complex and dependent key roles in starch degradation and biosynthesis pathways.
From a technological point of view, mono-phosphorylated starch has increased hydration capacity, thereby influencing starch pasting properties, gel strength and clarity, stickiness and viscosity18,19,20. Hence, phopshorylated starch can improve starch functionality and can be possibility produced directly in the cereal grain, which shows tremendous potential economic and environmental advantage2. Engineering the expression of GWD1 or PWD/GWD3 is the obvious strategy to alter starch phosphate in the crop. The manipulation of GWD1 activity in the crop has been described in some patents including the overexpression in wheat21 and corn22 leading to increased viscosity of the starch paste. Overexpression of the potato GWD1 gene specifically in the developing barley endosperm resulted in grain starch with ten-fold increased phosphate content, and starch granules showing altered morphology and lower melting enthalpy15, 16, 23.
Rice is a crucial cereal crop in developing countries and the milled grains are composed of approximately 80–90% starch24. Rice endosperm starch contains low concentrations of starch bound phosphate esters (≈1 nmol/mg)3, 8, which limits its usage in various industrial processes. Whether introduction of potato GWD1 gene into rice could produce starch with higher bound phosphate esters needs to be addressed, which may introduce starch phosphate into the smallest granule found among cereals. The objective of this study is to express the potato GWD1 gene in rice (japonica, cv Zhonghua 11) endosperm amyloplasts, and to investigate the morphological and physicochemical properties of the resulting modified rice starch.
Results
Production of transgenic GWD1 overexpression rice plants
To determine whether rice starch phosphate esterification could be increased by genetic modification and whether the starch functional properties could be improved to widen its application potential, we generated rice lines overexpressing the gene (StGWD1) coding for the potato starch phosphorylator GWD1. Stable transgenic rice lines were generated by Agrobacterium-mediated transformation using pUCED-Hord:TP-StGWD:NOS vector (Supplementary Fig. 1) that enables endosperm-specific overexpression of transgenic protein and targeting to the amyloplasts by an N-terminal transit peptide (TP)25. Sixteen transgenic lines among 24 candidate plants in the T0 generation were identified by genomic PCR for hygromycin phosphotransferase (HPT) and StGWD1 as selection markers (Supplementary Fig. 2A). These T0 lines were propagated by self-pollination for four generations to create homozygous transgenic lines (Supplementary Fig. 2B). Six stable individual transgenic lines were obtained, defined as RGD1-6, respectively (Supplementary Fig. 2C).
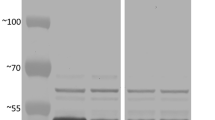
Measurements of expression of the StGWD1 gene in developing endosperm were carried out using qRT-PCR. All six selected transgenic lines of RGD1-6 had high expression levels of StGWD1 (Fig. 1A). The presence of StGWD1 protein in the developing endosperms was confirmed in the RGD1-6 lines by western blotting (Fig. 1B).
(A) Expression of StGWD1 in the developing endosperm of stable offspring lines of transgenic rice (RGD1-6) at the transcript level determined by qRT-PCR (up: StGWD1, down: actin), and (B) StGWD1 protein determined by western blotting (up: StGWD1, down: the total protein). The gels were cropped to only show the relevant bands. M: marker, 1: control, 2-7: RGD1-6.
Starch phosphate content
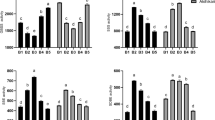
The contents of Glc-6-P and Glc-3-P units were very low in starch of the control cultivar Zhonghua11, 0.15 nmol/mg and 0.23 nmol/mg, respectively, corresponding to that only one of approx. 30 000–50 000 glucose units in the starch is phosphomonoesterified (Fig. 2A). The transgenic rice grains had 9-fold higher Glc-6-P content as compared to control (Fig. 2A). These data are consistent with the barley system expressing the same gene23. Also the Glc-3-P content was double fold higher in the transgenic lines than the control. This is also consistent with the activity of PWD/GWD3 being strictly dependent on pre-phosphorylated starch8, 9. The concentrations of Glc-6-P and Glc-3-P varied among the transgenic lines, the RGD-5 having the highest Glc-6-P content. This variation followed the expression of the StGWD1gene.
Starch granule morphology
The transgenic starch granules had similar polygonal shapes as control Zhonghua 11 (Fig. 3). However, some granules had blurred edges and more irregular surfaces, especially granules from lines RGD3 and RGD5. Some starch granules had pores visible at the surface. A similar observation was seen on starch granules from barley plants with overexpression of StGWD1 23 demonstrating a phosphate stimulated destabilization of the starch granules as proposed5.
Apparent amylose content (AAC)
The AAC was higher in all the transgenic lines as compared to control (Fig. 2B) and the Glc-6-P content was positively correlated with the AAC (r = 0.871, P < 0.05, Supplementary Table 2).
Pasting viscosity
Pasting characteristics of suspensions of 3.0 g flour in 25.0 g of distilled water were analyzed by RVA, and the result showed that the peak viscosity (PV), pasting temperature (PT), breakdown (BD) and setback (SB) were significantly lower for transgenic lines as compared to control (Table 1). The Glc-6-P and Glc-3-P content were negatively correlated with PT, PV and BD, but positively correlated with SB (Supplementary Table 2).Pasting viscosity is expected to be enhanced by the presence of starch phosphomonoesters19. Hence, these data suggest that the pasting properties of the flour from the transgenic lines may be due to the increased starch phosphates as well as higher AAC content in these lines since amylose is known to suppress starch pasting26.
Gel texture
The hardness of gels prepared from rice flour of RGD3-6 was significantly higher than that of the control. However, gels prepared from RGD1 and RGW2 flour had lower gel hardness than control (Table 2). The adhesiveness of RGD3 and RGD5 flour was significantly lower than that of the control. By contrast the cohesiveness of these lines was significantly higher (Table 2). The other transgenic lines had adhesiveness and cohesiveness similar to that of the control (Table 2). Starch phosphate is predicted to weaken the gel network27, while amylose content has been reported to be positively related to gel hardness28, 29, and the interplay between phosphate and amylose in paste and gel systems can be complex27, 30. For the transgenic rice lines, the higher amylose content can explain the increased hardness of GWD1 and GWD2 flour.
Differential scanning calorimetry
The dissolution temperature parameters To, Tp and Tc of transgenic starch in water were significantly lower than those of the control (Table 3). Also, the dissolution enthalpy, ΔH, of transgenic starch was significantly lower than control (Table 3). The degree of phosphorylation, i.e. the Glc-6-P and Glc-3-P content, was strongly negatively correlated with the gelatinization parameters To, TP and Tc (r = −0.871, −0.921 and −0.898, respectively) (Supplementary Table 3) while only very weak negative correlations were found for the ΔH parameter. For the barley system To, Tp and Tc are not different for highly phosphorylated transgenic barley lines as compared to the control, however ΔH is significantly lower23. For the potato system, the low starch phosphate contents from a GWD1 suppressor lines does not significantly affect the To, Tp and Tc but ΔH is increased, indicating disturbed crystalline perfection induced by phosphate esters5, 31. The decrease in dissolution temperatures with starch phosphorylation found in the rice system could be directly associated with phosphate-induced destabilization of crystalline starch in the granules. Since the effects of phosphate are seemingly not the same for different plant and organ systems, additional factors can have influence on starch granule dissolution including the amount and distribution of amylose, protein and lipids.
Chain length distribution
Surveying starch samples cross species, relations are found for the chain length and crystalline polymorphs of the starch granules4. Typically, phosphorylated starches have longer amylopectin chains than non-phosphorylated ones and highly phosphorylated starches specifically shows an increasing proportion of chains with at DP 194. To investigate if there is a similar effect in rice, the amylopectin chain length distribution was analyzed by HPAEC-PAD (Table 4). The unit chains of debranched amylopectin can be grouped into fa (DP 6-12), fb1 (DP 13-24), fb2 (DP 25-36) and fb3 (DP ≥ 37) populations32. The profiles of the control and transgenic lines were rather similar but specific changes could be detected. Generally, the average chain length of amylopectin from transgenic lines was higher, which is in agreement with the notion that phosphorylated chains in starch granule are typically longer, compared to non-phosphorylated amylopectin chains4. Compared to control, the short main peak DPs of transgenic lines were somewhat decreased (from DP15 to DP14) and the content of longer chains were increased in lines RGD1, RGD2 and RGD5. Hence, we found fewer chains in the fb1 (DP 13-24) population and more chains in the fb3 (DP ≥ 37) population as compared to control. Correlation analysis demonstrated that the Glc-6-P content was negatively correlated with the proportion of DP 13-14 (r = −0.863), but positively correlated with the proportion of DP 25-36 (r = 0.941) (Supplementary Table 3). The Glc-3-P content was positively correlated with the proportion of DP 6-12 (r = 0.840) (Supplementary Table 3). However, for the barley system expressing the StGWD1 gene, no significant relationship was found for amylopectin chain length and starch phosphorylation15 demonstrating the increasing the phosphorylation by overexpressing the GWD1 gene does not significantly affect the amylopectin chain structure.
Discussion
Potato glucan water dikinase 1 (StGWD1) is a 155 kDa protein, and is known to be involved in starch metabolism by adding phosphate groups to amylopectin33, 34. Homologs of StGWD1 have been found in many other plants and organs such as tubers, endosperms of cereals, fruits and leaves, demonstrating the high conservation of GWD135, 36. Several studies were made to alter the expression of GWD1 homologs in potato19, 31, barley23, maize37, and wheat12, and mutant analyses aimed at economically important traits, such as physicochemical properties of starch, starch content, and plant biomass. The endosperm-specific inhibition of GWD1 homologs in wheat by RNAi decreases the amount of grain starch-bound Glc-6-P by up 70%, and increases plant biomass12. Carciofi et al. reported about a 10-fold increase in grain starch-bound phosphate in barley by over-expressing of StGWD1 in endosperm amyloplasts, and the high starch phosphate content in these transgenic lines results in altered granule morphology and affects the physicochemical properties of starch23. In this study, we generated six stable individual transgenic lines over-expressing of StGWD1 in rice (japonica, cv. Zhonghua 11) endosperm, which lead to about 9-fold and double increase in Glc-6-P and Glc-3-P, respectively, consisting with the results of Carciofi et al.23. The degree of substitution for industrial, chemically modified monostarch phosphate is less than 0.5% (FAO, http://www.fao.org/docrep/w6355e/w6355e0o.htm) corresponding to approximately 50 nmol/mg. This level is in the same range as for highly phosphorylated potato starch5. For the chemically modified rice starch, the phosphate content was increased by 3-fold or 4-fold, which displayed significantly increased food freeze-thaw stability38. Although the phosphate content in the transgenic rice was not as high as highly phosphorylated potato, we suppose that the content of starch phosphate in these lines is in a concentration range sufficient for application where stabilized starch is required.
The starch granules of transgenic lines showed only minor morphologically differences including the presence of surface pores and slightly distorted edges and irregular surfaces found in some granules. Molecular force-field models indicate that a phosphate ester can be linked at the C-6 position to amylopectin without disturbing its double helical structure39, 40. The same models show that a phosphate ester at the C-3 position imparts molecular strain in the double helical motif thereby preventing optimal crystalline packing39, 40. Following studies indicated that starch phosphate esters can stimulate hydrolytic enzyme activity in vitro and have a stabilizing effect on the β-amylase-glucan ligand complex, supporting that starch phosphate esters can direct amylases to phosphorylated spots by GWD1 and PWD/GWD3 catalyzed on starch granules10, 41. Hence, the presence of pores in starches with GWD1 over-expressed rice lines indicates that the presence of GWD1 seems to stimulate starch degradation in rice endosperm.
GWD1 efficiently catalyzes the phosphorylation of crystalline maltodextrins and thereby induces the solubilization of both the neutral and the phosphorylated glucans in these crystalline aggregates17. Phosphorylation also influences the physicochemical properties of starch, i.e. decreases the melting enthalpy for starch as deduced from starch extracted from tubers of GWD1 antisense suppressed potato lines31. DSC data for the transgenic rice starch showed that the dissolution temperature parameters To, Tp and Tc and dissolution enthalpy (ΔH) of hyper-phosphorylated starch in GWD1 over-expression lines were significantly lower than control. Amylose is known to suppress ΔH31, so the suppression of ΔH can be a combined effect of increased amylose and starch phosphate. The low dissolution temperatures found can be an effect of phosphate-induced disturbance of the crystalline lattice of the granules even though this parameter was not affected in the potato system31. In the barley system To, Tp, Tc are not different for hyper-phosphorylated starch as compared to control but ΔH is decreased despite the constant amylose content23. Our correlation analysis for the rice system demonstrated that the degree of phosphorylation in GWD1 over-expressed rice lines was negative correlated with ΔH, but not at significant level. For potato tuber starch, a strong negative correlation has been found between melting enthalpy of the lintnerised starch and the phosphate content42. Hence, the effects of starch phosphate monoesters are apparently complex not the same for different starch granule systems. Factors including the amount and distribution of amylose, protein and lipids are also important.
The AAC was higher in all the transgenic lines as compared to control (Fig. 2B) and was positively correlated with Glc-6-P content (r = 0.871, P < 0.05, Supplementary Table 2). This is not in agreement with data for the barley system23, where amylose was not affected demonstrating different metabolic routes in these two plants. For the potato tuber system, decreased starch phosphate content, due to RNA interference suppression of the GWD1, resulted in increased amylose content19. Hence, the different effects on amylose content in the phosphorylation-modified rice, barley and potato models are not consistent and needs further investigation, e.g. quantification of the amylose synthesising enzyme such as granule bound starch synthase. Pasting profiles using RVA can reveal effects of specific molecular structures, including phosphate monoesters and amylose, on starch granule swelling and disintegration of starch granules during heating and shear43. Especially the degree of phosphorylation is known to enhance the pasting capability of starch18, 19. It is reported that the pasting viscosity parameters are highly correlated with the AAC for rice starches26, 44. Our data showed that the AAC was negatively correlated with PV and CPV, while both G-6-P and G-3-P had significant negative correlation with most RVA parameters, notably PV and BD, but positively affected the SB and the gel hardness (Supplementary Table 2). The latter effects may be due to the combined high amylose and dynamics of phosphate-stimulating chain re-association to efficiently form an entangled gel network. Provided that the starch gelatinizes well, higher amylose content and longer amylopectin chains tend to exhibit harder gels29. The general negative effect on pasting behavior (except for the SB) found in the rice system is supposedly directed mainly by the high amylose content and less by the increased phosphate.
Phosphorylated starches are of tremendous value for technical applications, and this type of modification yields a significant hydration capacity producing clear and highly viscous starch pastes19, 45, 46. If phosphorylated starch modification carried out directly in the crop by transgenic biotechnology, modern breeding or mutagenesis, starch functionality can be improved with little or no requirement for post-harvest processing, which has tremendous potential economic and environmental advantages2. We here produced hyper-phosphorylated starch in rice by endosperm-specific transgenic overexpression of potato StGWD1 resulting in an increase of starch phosphate content and the improvement of starch functionality. Our data demonstrate a way forward to employ a starch bioengineering approach for clean modification of starch.
Conclusion
Rice starch contains only low concentrations of starch bound phosphate monoesters, which limits its usage in various industrial processes. We produced six stable individual transgenic lines with hyper-phosphorylated starch by the overexpression of the StGWD1 in rice (japonica, cv Zhonghua 11). The transgenic lines had 9-fold and double higher Glc-6-P and Glc-3-P, respectively and increased amylose content. The starch granules displayed only minor morphological alterations, notably the presence of surface pores and moderately distorted edges and surfaces. The novel starch introduces unique combinations of functionality for rice starch, such as reduced gelatinization temperature, decreased pasting viscosity, increased gel formation capacity and increased gel hardness.
Methods
Plant materials
Rice japonica variety “Zhonghua11” (O. sativa L.) was used as the parent cultivar. The plants were grown in the greenhouse at the Zhejiang University, Huajiachi Campus, Hangzhou, China. The developing seeds were harvested 15 days after flowering (DAF), immediately frozen in liquid nitrogen and stored at −80 °C. Mature seeds were harvested 40 DAF for extraction of starch and analysis of physicochemical properties.
Transformation
The plasmid vector pUCED-Hord:TP-StGWD:NOS was the same as that for the transformation of barley (Supplementary Fig. 1)23, 25. D-hordein promoters have earlier been demonstrated to direct strong endosperm specific gene expression47. Transformation of rice was performed according to the method of Duan et al.48.
DNA and RNA extraction, and PCR amplification
Total genomic DNA was extracted from fresh leaves using modified cetyltrimethylammonium bromide (CTAB) method49. Putative transgenic plants were identified for the presence of the transgene by PCR amplification. Specific PCR primers were designed using the Primer Premier 5.0 software, according to the StGWD1 gene and HPT gene sequence (Supplementary Table 1). The PCR reaction was performed in a volume of 10 μL containing 5 μL 2× PCR Mix buffer (ToYoBo), 50 ng genomic DNA samples and 1 μL forward and reverse primers (10 μM). PCR amplification procedure was 4 min at 94 °C followed by 35 cycles of 1 min at 94 °C, 1 min at 57 °C and 30 s at 72 °C. The PCR products were checked for the presence of fragment by agarose gel electrophoresis.
Total RNA was extracted using UNIQ-10 Column Total RNA Purification Kit (Sangon Biotech) and then reverse transcribed to cDNA using Frist-Strand Synthesis of cDNA kit (Promega) according to the manufacturer’s instructions. The specific primers used in the subsequent RT-PCR are listed in Supplementary Table 1. Actin was used as internal housekeeping control for normalization of mRNA content. The conditions and procedures for RT-PCR was the same as for standard PCR described above.
Western Blotting
Soluble proteins were extracted according to Chen and Bao50, their concentration measured using NANODROP 2000 spectrophotometer (Thermo, Canada) and sodiumdodecyl sulphate polyacrylamide electrophoresis (SDS-PAGE)51. Separated proteins were blotted onto polyvinylidene fluoride fluoride (PVDF) membranes using a transblotter and GWD1 was detected using western blotting according to Crofts et al.52 using rabbit polyclonal antiserum directed against StGWD1 as probe20.
Preparation of rice flour and starch
The mature rice grains were sun-dried until the seed moisture was around 12%, and stored at room temperature for three months28. Rice flour preparation was according to Bao et al.28 and starch was extracted according to Kong et al.26.
Scanning electron microscopy (SEM)
The starch samples were attached to specimen stub and sputter coated with gold using Eiko IB5 before viewing with scanning electron microscope (TM-1000).
Starch phosphate content and apparent amylose content (AAC)
G-6-P and G-3-P contents were quantified using high-performanceanion-exchange chromatography (HPAEC) with pulsed amperometric detection (PAD)53. AAC was measured using the iodine staining method28, calculated using a standard curve made from 4 rice samples with known amylose content.
RVA pasting properties
The paste property of rice flour was determined byusing a Rapid Visco Analyzer (RVA-3, Newport Scientific, Warriewood, Australia). According to the method of AACC61-02, 3.0 g of rice flour was placed an aluminum canister, and 25.0 g ofdistilled water was added. The heating profile was: (1) constant 50 °C for 1 min; (2) linear ramp to 95 °C until 4.8 min; (3) constant 95 °C until 7.3 min; (4) linear ramp down to 50 °C at 11.1 min and (5) constant 50 °C until 12.5 min. Three primary parameters were calculated from the pasting curves: peak viscosity (PV), hot paste viscosity (HPV), and cool paste viscosity (CPV). Three secondary parameters were calculated from primary parameters: breakdown viscosity (BD = PV − HPV), setback viscosity (SB = CPV − PV) and consistency viscosity (CS = CPV − HPV). The viscosity parameters were measured in Rapid Visco Units (RVU). Pasting temperature (PT) was determined according to the method proposed by Bao et al.28.
Gel texture
The sample cans containing rice starch gels formed in RVA analysis were sealed by Parafilm and kept at 4 °C for 24 h. Textural properties were determined by a TA-XT2i Texture Analyzer (Texture Technologies Corp., Scarsdale, NY) equipped with Texture Expert software (version 1.2) in a two-cycle programme. Hardness, adhesiveness, and cohesiveness were calculated using the Texture Expert software program (Version 5.16).
Thermal properties
Thermal properties were monitored using a Differential Scanning Calorimeter model Q20 (TA Instruments, New Castle, DE, USA), and the parameters of Onset (To), Peak (Tp), Conclusion temperatures (Tc) and enthalpy (ΔHg) of gelatinization were calculated by a Universal Analysis Program, version 4.4 A (TA instruments, Newcastle, DE, USA).
Chain-Length Distribution Determination
The distribution of the amylopectin side chains was performed following enzymatic debranching using isoamylase and separation of the generated linear fragments using HPAEC-PAD54.
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) and Tukey’s test to determine significant differences by SAS statistical software (version 9.3, SAS Institute Inc., Cary, NC). Correlation analyses among the different parameters were calculated by the Proc Corr procedure.
References
Light, J. M. Modified food starches: why, what, where and how. Cereal Foods World 35, 1081–1092 (1990).
Hebelstrup, K. H., Sagnelli, D. & Blennow, A. The future of starch bioengineering: GM microorganisms or GM plants? Front Plant Sci. 6, 247 (2015).
Fernbach, A. Some observations on the composition of potato starch. C. R. Acad. Sci. 138, 428–430 (1904).
Blennow, A., Engelsen, S. B., Munck, L. & Møller, B. L. Starch molecular structure and phosphorylation investigated by a combined chromatographic and chemometric approach. Carbohyd. Polym. 41(2), 163–174 (2000).
Blennow, A. & Engelsen, S. B. Helix-breaking news: fighting crystalline starch energy deposits in the cell. Trends Plant Sci. 15(4), 236–240 (2010).
Bay-Smidt, A. M., Wischmann, B., Olsen, C. E. & Nielsen, T. H. Starch bound phosphate in potato as studied by a simple method for determination of organic phosphate and (31) P-NMR. Starch-Stärke 46(5), 167–172 (1994).
Ritte, G. et al. Phosphorylation of C6-and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett. 580(20), 4872–4876 (2006).
Baunsgaard, L. et al. A novel isoform of glucan, water dikinase phosphorylates pre- phosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J. 41(4), 595–605 (2005).
Kötting, O. et al. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol. 137(1), 242–252 (2005).
Edner, C. et al. Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial β-amylases. Plant Physiol. 145(1), 17–28 (2007).
Mahlow, S. et al. Phosphorylation of transitory starch by α-glucan, water dikinase during starch turnover affects the surface properties and morphology of starch granules. New Phytol. 203(2), 495–507 (2014).
Ral, J. P. et al. Down-regulation of glucan, water-dikinase activity in wheat endosperm increases vegetative biomass and yield. Plant Biotechnol. J. 10(7), 871–882 (2012).
Yu, T. S. et al. The Arabidopsissex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13(8), 1907–1918 (2001).
Skeffington, A. W., Graf, A., Duxbury, Z., Gruissem, W. & Smith, A. M. Glucan, water dikinase exerts little control over starch degradation in Arabidopsis leaves at night. Plant Physiol. 165, 866–879 (2014).
Shaik, S. S. et al. Alterations in starch branching enzyme and glucan water dikinase: Effects on grain physiology and metabolism. PloS ONE 11(2), e0149613 (2016).
Shaik, S. S., Carciofi, M., Martens, H. J., Hebelstrup, K. H. & Blennow, A. Starch bioengineering affects cereal grain germination and seedling establishment. J. Exp. Bot. 65(9), 2257–2270 (2014).
Hejazi, M. et al. Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J. 55(2), 323–334 (2008).
Wiesenborn, D. P., Orr, P. H., Casper, H. H. & Tacke, B. K. Potato starch paste behavior as related to some physical/chemical properties. J. Food Sci. 59(3), 644–648 (1994).
Viksø-Nielsen, A. et al. Structural, Physicochemical, and pasting properties of starches from potato plants with repressed r 1-gene. Biomacromolecules 2(3), 836–843 (2001).
Mikkelsen, R., Baunsgaard, L. & Blennow, A. Functional characterization of alpha-glucan, water dikinase, the starch phosphorylating enzyme. Biochem. J. 377(2), 525–532 (2004).
Schewe, G. et al. Patent No. 6,734,340. Washington, DC: U.S. Patent and Trademark Office (2004).
Lanahan, M. & Basu, S. Modified starch, uses, methods for production thereof: U.S. Patent Application 10/556,276 (2004).
Carciofi, M. et al. Hyperphosphorylation of cereal starch. J. Cereal. Sci. 54(3), 339–346 (2011).
Vandeputte, G. E. & Delcour, J. A. From sucrose to starch granule to starch physical behaviour: a focus on rice starch. Carbohyd. Polym. 58(3), 245–266 (2004).
Hebelstrup, K. H. et al. UCE: A uracil excision (USER™)-based toolbox for transformation of cereals. Plant Methods 6(1), 1–10 (2010).
Kong, X. L., Zhu, P., Sui, Z. Q. & Bao, J. S. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 172, 433–440 (2015).
Blennow, A. et al. Structure-function relationships of transgenic starches with engineered phosphate substitution and starch branching. Int. J. Biol. Macromol. 36, 159–168 (2005).
Bao, J. S., Shen, S. Q., Sun, M. & Corke, H. Analysis of genotypic diversity in the starch physicochemical properties of nonwaxy rice: apparent amylose content, pasting viscosity and gel texture. Starch-Stärke 58(6), 259–267 (2006).
Wang, L. et al. Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocolloids 24(2), 208–216 (2010).
Blennow, A., Bay-Smidt, A. M., Leonhardt, P., Bandsholm, O. & Madsen, H. M. Starch paste stickiness is a relevant native starch selection criterion for wet-end paper manufacturing. Starch-Stärke 55, 381–389 (2003).
Kozlov, S. S., Blennow, A., Krivandin, A. V. & Yuryev, V. P. Structural and thermodynamic properties of starches extracted from GBSS and GWD suppressed potato lines. Int. J. Biol. Macromol. 40(5), 449–460 (2007).
Hanashiro, I., Abe, J. & Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 283, 151–159 (1996).
Mikkelsen, R. & Blennow, A. Functional domain organization of the potato alpha-glucan, water dikinase (GWD): evidence for separate site catalysis as revealed by limited proteolysis and deletion mutants. Biochem. J. 385, 355–361 (2005).
Zeeman, S. C., Kossmann, J. & Smith, A. M. Starch: its metabolism, evolution, and biotechnological modification in plants. Plant Biol. 61(1), 209–234 (2010).
Ritte, G., Eckermann, N., Haebel, S., Lorberth, R. & Steup, M. Compartmentation of the starch-related R1 protein in higher plants. Starch- Stärke 52(5), 145–149 (2000).
Mikkelsen, R., Mutenda, K. E., Mant, A., Schürmann, P. & Blennow, A. α-Glucan, water dikinase (GWD): A plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc. Natl. Acad. Sci. USA 102(5), 1785–1790 (2005).
Weise, S. E. et al. Engineering starch accumulation by manipulation of phosphate metabolism of starch. Plant Biotechnol. J. 10(5), 545–554 (2012).
Deetae, P. et al. Preparation, pasting properties and freeze-thaw stability of dual modified crosslink-phosphorylated rice starch. Carbohyd. Polym. 73, 351–358 (2008).
Blennow, A., Nielsen, T. H., Baunsgaard, L., Mikkelsen, R. & Engelsen, S. B. Starch phosphorylation: a new front line in starch research. Trends Plant Sci. 7(10), 445–450 (2002).
Engelsen, S. B. et al. The phosphorylation site in double helical amylopectin as investigated by a combined approach using chemical synthesis, crystallography and molecular modeling. FEBS Lett. 541(1–3), 137–144 (2003).
Dudkiewicz, M., Simińska, J., Pawłowski, K. & Orzechowski, S. Bioinformatics analysis of oligosaccharide phosphorylation effect on the stabilization of the β-amylase ligand complex. J. Carbohyd. Chem. 27(8–9), 479–495 (2008).
Wikman, J. et al. Influence of amylopectin structure and degree of phosphorylation on the molecular composition of potato starch lintners. Biopolymers 101(3), 257–271 (2014).
Batey, I. L., Crosbie, G. B. & Ross, A. S. Interpretation of RVA curves. The RVA Handbook 19–30 (2007).
Bao, J. S., Sun, M. & Corke, H. Analysis of genotypic diversity in starch thermal and retrogradation properties in nonwaxy rice. Carbohyd. Polym. 67(2), 174–181 (2007).
Thygesen, L. G., Blennow, A. & Engelsen, S. B. The effects of amylose and starch phosphate on starch gel retrogradation studied by low-field 1H NMR relaxometry. Starch-Stärke 55(6), 241–249 (2003).
Jobling, S. Improving starch for food and industrial applications. Curr. Opinion Plant Biol. 7(2), 210–218 (2004).
Horvath, H. et al. The production of recombinant proteins in transgenic barley grains. Proc. Natl. Acad. Sci. U.S.A. 97(4), 1914–1919 (2000).
Duan, Y. et al. An efficient and high-throughput protocol for agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in japonica rice (Oryza sativa L.). Plant Cell Rep. 31, 1611–1624 (2012).
Murray, M. G. & Thompson, W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8(19), 4321–4326 (1980).
Chen, Y. L. & Bao, J. S. Underlying mechanisms of zymographic diversity in starch synthase I and pullulanase in rice-developing endosperm. J. Agric. Food Chem. 64(9), 2030–2037 (2016).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259), 680–685 (1970).
Crofts, N. et al. Lack of starch synthase IIIa and high expression of granule-bound starch synthase I synergistically increase the apparent amylose content in rice endosperm. Plant Sci. 193, 62–69 (2012).
Blennow, A., Bay-Smidt, A. M., Olsen, C. E. & Møller, B. L. Analysis of starch-bound glucose 3-phosphate and glucose 6-phosphate using controlled acid treatment combined with high-performance anion-exchange chromatography. J. Chromatogr. A 829, 385–391 (1998).
Blennow, A., Bay-Smidt, A. M., Wischmann, B., Olsen, C. E. & Møller, B. L. The degree of starch phosphorylation is related to the chain length distribution of the neutral and the phosphorylated chains of amylopectin. Carbohyd. Res. 307(1), 45–54 (1998).
Acknowledgements
This work was financially supported by the National Key Research and Development Program (2016YFD0400104), and the Fundamental Research Funds for the Central Universities at Zhejiang University, Hangzhou, China (Grant No. 2016XZZX001-09).
Author information
Authors and Affiliations
Contributions
J.S.B. and A.B. designed the experiments, Y.C., X.S. and X.Z. performed the experiments, K.H.H., A.B. and J.S.B. analyzed the data, Y.C., A.B. and J.S.B. wrote manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Sun, X., Zhou, X. et al. Highly phosphorylated functionalized rice starch produced by transgenic rice expressing the potato GWD1 gene. Sci Rep 7, 3339 (2017). https://doi.org/10.1038/s41598-017-03637-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03637-5
This article is cited by
-
Effects of phosphorylation on the chemical composition, molecular structure, and paste properties of Hedychium coronarium starch
Food and Bioprocess Technology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.