Abstract
Modeling immuno-oncology by using patient-derived material and immune cell co-cultures can advance our understanding of immune cell tumor targeting in a patient-specific manner, offering leads to improve cellular immunotherapy. However, fully exploiting these living cultures requires analysis of the dynamic cellular features modeled, for which protocols are currently limited. Here, we describe the application of BEHAV3D, a platform that implements multi-color live 3D imaging and computational tools for: (i) analyzing tumor death dynamics at both single-organoid or cell and population levels, (ii) classifying T cell behavior and (iii) producing data-informed 3D images and videos for visual inspection and further insight into obtained results. Together, this enables a refined assessment of how solid and liquid tumors respond to cellular immunotherapy, critically capturing both inter- and intratumoral heterogeneity in treatment response. In addition, BEHAV3D uncovers T cell behavior involved in tumor targeting, offering insight into their mode of action. Our pipeline thereby has strong implications for comparing, prioritizing and improving immunotherapy products by highlighting the behavioral differences between individual tumor donors, distinct T cell therapy concepts or subpopulations. The protocol describes critical wet lab steps, including co-culture preparations and fast 3D imaging with live cell dyes, a segmentation-based image processing tool to track individual organoids, tumor and immune cells and an analytical pipeline for behavioral profiling. This 1-week protocol, accessible to users with basic cell culture, imaging and programming expertise, can easily be adapted to any type of co-culture to visualize and exploit cell behavior, having far-reaching implications for the immuno-oncology field and beyond.
Key points
-
BEHAV3D is a 3D live imaging platform for the analysis of engineered T cell behavior and tumor response. It enables assessment of inter- and intratumoral heterogeneity in response to cellular immunotherapy and the discovery of T cell modes of action associated with this heterogeneity.
-
This approach is unique because it combines monitoring the response of patient-specific tumor cells or organoids with tracking of large numbers of therapeutic T cell populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data described in this protocol are available from the corresponding author upon reasonable request. Representative raw imaging datasets used for PDO Behavioral map generation can be found on BioImage Achive under accession no. S-BIAD448, and representative processed imaging datasets can be found on Dryad (https://datadryad.org/stash/dataset/doi:10.5061/dryad.3n5tb2rp6). Analyzed demo data are made publicly available through GitHub (https://github.com/AlievaRios/BEHAV3D/tree/main/demos).
Code availability
All source codes of BEHAV3D are publicly available through GitHub (https://github.com/AlievaRios/BEHAV3D).
References
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Irvine, D. J., Maus, M. V., Mooney, D. J. & Wong, W. W. The future of engineered immune cell therapies. Science 378, 853–858 (2022).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Ooft, S. N. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 11, eaay2574 (2019).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Liu, C., Yang, M., Zhang, D., Chen, M. & Zhu, D. Clinical cancer immunotherapy: current progress and prospects. Front. Immunol. 13, 961805 (2022).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2022).
Jacob, F., Ming, G. & Song, H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat. Protoc. 15, 4000–4033 (2020).
Cattaneo, C. M. et al. Tumor organoid–T-cell coculture systems. Nat. Protoc. 15, 15–39 (2020).
Schnalzger, T. E. et al. 3D model for CAR‐mediated cytotoxicity using patient‐derived colorectal cancer organoids. EMBO J. 38, e100928 (2019).
Freckmann, E. C. et al. Traject3d allows label-free identification of distinct co-occurring phenotypes within 3D culture by live imaging. Nat. Commun. 13, 5317 (2022).
Matthews, J. M. et al. OrganoID: a versatile deep learning platform for tracking and analysis of single-organoid dynamics. PLoS Comput. Biol. 18, e1010584 (2022).
Bandey, I. N. et al. Designed improvement to T-cell immunotherapy by multidimensional single cell profiling. J. Immunother. Cancer 9, e001877 (2021).
Romain, G. et al. Multidimensional single-cell analysis identifies a role for CD2-CD58 interactions in clinical antitumor T cell responses. J. Clin. Invest. 132, e159402 (2022).
Crainiciuc, G. et al. Behavioural immune landscapes of inflammation. Nature 601, 415–421 (2022).
Upadhaya, S., Hubbard-Lucey, V. M. & Yu, J. X. Immuno-oncology drug development forges on despite COVID-19. Nat. Rev. Drug Discov. 19, 751–752 (2020).
Voabil, P. et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nat. Med. 27, 1250–1261 (2021).
Kantari-Mimoun, C. et al. CAR T-cell entry into tumor islets is a two-step process dependent on IFNγ and ICAM-1. Cancer Immunol. Res. 9, 1425–1438 (2021).
You, R. et al. Active surveillance characterizes human intratumoral T cell exhaustion. J. Clin. Invest. 131, e144353 (2021).
Wang, Z. et al. Rapid tissue prototyping with micro-organospheres. Stem Cell Rep. 17, 1959–1975 (2022).
Ding, S. et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell 29, 905–917.e6 (2022).
Khazen, R. et al. Functional heterogeneity of cytotoxic T cells and tumor resistance to cytotoxic hits limit anti‐tumor activity in vivo. EMBO J. 40, e106658 (2021).
Cazaux, M. et al. Single-cell imaging of CAR T cell activity in vivo reveals extensive functional and anatomical heterogeneity. J. Exp. Med. 216, 1038–1049 (2019).
Weigelin, B. et al. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 12, 5217 (2021).
Ineveld et al. Multispectral confocal 3D imaging of intact healthy and tumor tissue using mLSR-3D. Nat. Protoc. 17, 3028–3055 (2022).
Bayarmagnai, B., Perrin, L., Pourfarhangi, K. E. & Gligorijevic, B. Intravital imaging of tumor cell motility in the tumor microenvironment context. Methods Mol. Biol. 1749, 175–193 (2018).
Kimmel, J. C., Chang, A. Y., Brack, A. S. & Marshall, W. F. Inferring cell state by quantitative motility analysis reveals a dynamic state system and broken detailed balance. PLoS Comput. Biol. 14, e1005927 (2018).
Tormene, P., Giorgino, T., Quaglini, S. & Stefanelli, M. Matching incomplete time series with dynamic time warping: an algorithm and an application to post-stroke rehabilitation. Artif. Intell. Med. 45, 11–34 (2009).
Gründer, C. et al. γ9 and δ2CDR3 domains regulate functional avidity of T cells harboring γ9δ2TCRs. Blood 120, 5153–5162 (2012).
Kuball, J. et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109, 2331–2338 (2006).
Wallstabe, L. et al. ROR1-CAR T-cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight 4, e126345 (2019).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Deben, C. et al. OrBITS: label-free and time-lapse monitoring of patient derived organoids for advanced drug screening. Cell Oncol. (Dordr.) 46, 299–314 (2023).
Dekkers, J. F. et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 16, 1936–1965 (2021).
Acknowledgements
We thank the Princess Máxima Center for Pediatric Oncology for technical support and the Hubrecht Institute and Zeiss for imaging support and collaboration. All imaging was performed at the Princess Máxima Imaging Center. We thank Hubrecht Organoid Technology for providing BC PDOs and the Princess Máxima Center Organoid Facility for organoid culture support. We thank E. Bokobza and M. Buchholz for providing the healthy breast organoid lines, D. van Vuurden (Princess Máxima Center for Pediatric Oncology) for providing DMG cultures, M. Koomen (Hubrecht Institute) for support with H&N cancer PDO culture and members of the Dream3DLAB (Rios group) for offering critical feedback on the project. This work was financially supported by the Princess Máxima Center for Pediatric Oncology. M.A. is supported by the Comunidad de Madrid (2022-T1/BMD-24021). J.F.D. was supported by a KWF Young Investigator grant (no. 14135). A.C.R. is supported by an ERC-starting grant 2018 project (no. 804412). M.B.R. and N.M.M.D. and the imaging work are supported by the Dutch ODAS Foundation.
Author information
Authors and Affiliations
Contributions
M.A. designed and performed the computational analysis. J.F.D., M.B.R. and D.P., set up co-cultures and performed imaging experiments, with assistance from A.L.Z. A.L.Z. cultured BC PDOs. M.A. and S.d.B. analyzed data. S.d.B. further adapted the computational workflow. M.B.R. assisted with imaging data processing. J.F.D. optimized experimental conditions for BC-PDO experiments, and M.B.R. optimized experimental conditions for liquid tumor experiments with supervision from J.F.D. A.M.C. and S.N. provided ROR1-CAR T cells. A.M.C., C.v.d.V. and N.M.M.D. generated and cultured CD19 cells. M.L.d.B. provided the ALL cells. R.P., S.N., F.G.J.C. and C.v.d.V. supervised the work with liquid tumors. Z.S. and J.K. provided the TEG cells and supervised work with these cells. H.C. supervised work with organoids. M.A., J.F.D. and A.C.R. designed the study. M.A. wrote the manuscript, with support from J.F.D., M.B.R., A.L.Z., D.P., A.C.R. and E.J.W. All authors critically revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.C. and J.F.D. are named as inventors on (pending) patents related to the organoid technology. For the full disclosure of H.C., see https://www.uu.nl/staff/JCClevers/Additional%20functions. Z.S. and J.K. are inventors on different patents for γδ TCR sequences, recognition mechanisms and isolation strategies. J.K. is a scientific cofounder and shareholder of Gadeta (www.gadeta.nl). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Navin Varadarajan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Dekkers, J. F. et al. Nat. Biotechnol. 41, 60–69 (2022): https://doi.org/10.1038/s41587-022-01397-w
Extended data
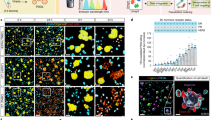
Extended Data Fig. 1 Organoid harvesting and co-culture setup.
a, Quantification of PDO targeting by using a CellTiter-Glo viability assay upon 24-h co-culture of organoids with TEGs with different cell culture media. b and c, Representative images of confluent BC PDO cultures (b) and BC PDO cultures plated too sparsely, plated too densely, with clear presence of cell debris or with good density (c). Scale bars, 100 μm. Numbers in the left corner of the images in b and c refer to specific BC PDO cultures (described in table 1 of ref. 38). d, Representative images of BME drops in a 12-well plate. Scale bar, 5 mm. Panels b–d reproduced with permission from ref. 38, Springer Nature America, Inc.
Extended Data Fig. 2 Specifics of the different live cell fluorescent dyes.
Representative images of distinct dye specific characteristics as described in Table 1. a, Living cells (yellow) present a dim dye eFluor 450 staining (green arrowheads), whereas dead cells (red) appear brighter (purple arrows). The gray arrowhead indicates an example of a non-cell-specific staining. b, Representative 3D images showing specifics of calcein AM. Viable cells are exclusively stained with this dye (white arrows), whereas dead cells can no longer retain this dye (purple arrows). c, Representative 3D images showing a main property of CellTracker Orange CMRA dye. Dead cells can also retain this dye (purple arrows), which holds true for all Cell Tracker dyes from Table 1. The gray arrowhead indicates debris. d, Representative optical sections showing specifics of CellBrite Steady 650. Cells exhibit a more membranal staining at the start of culture (gray arrow), which transitions into a preponderantly cytoplasmic staining at later time points (white arrow). e, Representative 3D images depicting specifics of Annexin V-CF555. Contrary to the manufacturer’s recommendations, no prelabeling of the dead cells is required, because a bright signal in the Annexin V-CF555 channel can be detected upon direct addition to the imaging medium (white arrowheads). Scale bars, 10 µm.
Extended Data Fig. 3 BEHAV3D imaging on SP8.
a, Representative 3D multispectral images of ALL tumor cells (yellow) in co-culture with CD8+ (blue) and CD4+ (green) CAR19 cells, showing tumor response (red) at the indicated time points. Scale bars, 100 µm. b and c, Single channels showing raw images (b) and corrected images after Step 37 (c) for CD8+ CAR19 (top) and CD4 CAR19 (bottom) channels.
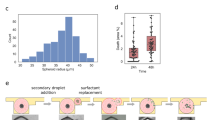
Extended Data Fig. 4 Example of the BEHAV3D image-processing procedure.
a, Representative overlayed and single-channel images of an example dataset consisting of PDOs in co-culture with CD4+ and CD8+ TEGs. These images are used as input for Step 38 explained in b. b and c, Channel arithmetic’s procedure and formulas described in Step 38 (b) that generate new image channels with cell-specific signal (c). d, Schematic representation of T cell segmentation and tracking in Steps 39 and 40. Channels 7 or 8 are taken as input for either CD8+ T cell or CD4+ T cell tracking. An autoregressive motion algorithm is used for tracking. e, Schematic representation of organoid segmentation in Step 41A for population-based death dynamics monitoring. f, Schematic representation of single organoid tracking in Step 41B. ROIs are defined around each individual organoid to facilitate their separation. A connected components algorithm is used for tracking. g, Optionally, distance transformation in Step 42 can be used to generate a new channel that represents the distance to organoid surfaces. h, T cell and tumor cell dynamic statistics are exported. The yellow boxes indicate the statistics that have to be selected in the statistics tab.
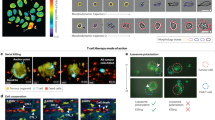
Extended Data Fig. 5 BEHAV3D imaging of TEGs targeting H&N and DMG tumors.
a, Representative 3D multispectral images of H&N tumor organoids (yellow) in co-culture with TEG cells (blue), showing the organoids’ response (red) at the indicated time points. b, Representative 3D multispectral images of DMG tumor organoids (yellow) in co-culture with TEG cells (blue), showing the organoids’ response (red) at the indicated time points. Scale bars, 100 µm.
Supplementary information
Supplementary Information
Supplementary Methods and Figs. 1 and 2
Supplementary Video 1
Representative video of an imaging experiment performed on the Zeiss LSM 880. The left panel displays raw imaging data of a co-culture of CD8+ CAR19 cells (blue), CD4+ CAR19 cells (green) and ALL cells (yellow). Dead dye presence is visualized in red. The right panel displays segmented and tracked data for CD8+ CAR19 cells (blue), CD4+ CAR19 cells (green) and ALL cells (gray). The track of the CAR19 cells represents the cell path over the last hour.
Supplementary Video 2
Representative video of an imaging experiment performed on the Leica SP8. The left panel displays raw imaging data of a co-culture of CD8+ CAR19 cells (blue), CD4+ CAR19 cells (green) and ALL cells (yellow). Dead dye presence is visualized in red. The right panel displays segmented and tracked data for CD8+ CAR19 cells (blue), CD4+ CAR19 cells (green) and ALL cells (gray). The track of the CAR19 cells represents the cell path over the last hour.
Supplementary Video 3
Representative video showing one single TEG cell, displaying morphological plasticity and destroying an entire tumor organoid in over 10 h. The left panel displays raw imaging data with TEG cells (blue) and a BC tumor organoid (yellow). Dead dye presence is visualized in red. The right panel displays segmented and tracked TEG cells (blue) and a BC tumor organoid (gray). The full T cell track is shown in blue.
Supplementary Video 4
Representative video showing one single motile CD8+ CAR19 cell (outlined in purple) performing five tumor cell killings in 8 h. CD8+ CAR19 cells (blue), CD4+ CAR19 cells (green) and ALL cells (yellow). Dead dye presence is visualized in red.
Supplementary Table 1
Table to quantify tracking accuracy metrics
Supplementary Table 2
BEHAV3D metadata template for organoid data processing
Supplementary Table 3
BEHAV3D metadata template for T cell data processing
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alieva, M., Barrera Román, M., de Blank, S. et al. BEHAV3D: a 3D live imaging platform for comprehensive analysis of engineered T cell behavior and tumor response. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00972-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-00972-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.