Abstract
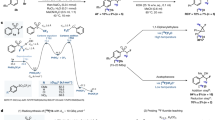
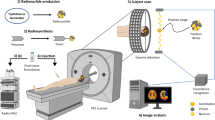
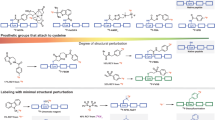
Positron emission tomography (PET) is a quickly expanding, non-invasive molecular imaging technology, and there is high demand for new specific imaging probes. Herein, we present a generic protocol for direct radiolabeling of heat-sensitive biomolecules with the positron-emitting radioisotope fluorine-18 (18F) using the aluminum fluoride restrained complexing agent (Al18F-RESCA) method. The Al18F-RESCA method combines the chemical advantages of a chelator-based radiolabeling method with the unique physical properties of the radionuclide of choice, fluorine-18. Proteins of interest can be conjugated to RESCA via amine coupling using (±)-H3RESCA-TFP, followed by purification using size-exclusion chromatography (SEC). Next, RESCA-derivatized biomolecules can be labeled in one step, at room temperature (~20 °C) in an aqueous medium with aluminum fluoride (Al18F). Al18F-labeled proteins can be obtained with moderate (12–17 GBq/µmol) to good (80–85 GBq/µmol) apparent molar activity, depending on the starting activity of 18F–. In addition, satisfactory radiochemical yields (35–55%, non–decay corrected) and high radiochemical purity (>98%, using gel filtration or solid-phase purification) are obtained. The mild radiolabeling procedure takes 0.5 h to complete and can be used for direct labeling of vector molecules such as peptides, protein scaffolds, and engineered antibody fragments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Naumova, A. V., Modo, M., Moore, A., Murry, C. E. & Frank, J. A. Clinical imaging in regenerative medicine. Nat. Biotechnol. 32, 804–818 (2014).
Serdons, K., Verbruggen, A. & Bormans, G. Developing new molecular imaging probes for PET. Methods 48, 104–111 (2009).
Bateman, T. M. Advantages and disadvantages of PET and SPECT in a busy clinical practice. J. Nucl. Cardiol. 19, S3–S11 (2012).
Le Bars, D. Fluorine-18 and medical imaging: radiopharmaceuticals for positron emission tomography. J. Fluor. Chem. 127, 1488–1493 (2006).
Sanchez-Crespo, A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 76, 55–62 (2013).
Smith, G. E., Sladen, H. L., Biagini, S. C. G. & Blower, P. J. Inorganic approaches for radiolabelling biomolecules with fluorine-18 for imaging with positron emission tomography. Dalton Trans. 40, 6196–6205 (2011).
Krishnan, H., Ma, L., Vasdev, N. & Liang, S. H. 18F-labeling of sensitive biomolecules for positron emission tomography. Chemistry 23, 15553–15577 (2017).
Jacobson, O., Kiesewetter, D. O. & Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 26, 1–18 (2015).
Richter, S. et al. Rerouting the metabolic pathway of 18F-labeled peptides: the influence of prosthetic groups. Bioconjug. Chem. 26, 201–212 (2015).
Thonon, D. et al. Fully automated preparation and conjugation of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) with RGD peptide using a GE FASTlab™ synthesizer. Mol. Imaging Biol. 13, 1088–1095 (2011).
Olberg, D. E. et al. One step radiosynthesis of 6-[(18)F]fluoronicotinic acid 2,3,5,6-tetrafluorophenyl ester ([[18F]F-Py-TFP): a new prosthetic group for efficient labeling of biomolecules with fluorine-18. J. Med. Chem. 53(4), 1732–1740 (2010).
Wängler, C. et al. One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc. 7, 1946–1955 (2012).
Liu, Z. et al. One-step 18F-labeling of biomolecules using organotrifluoroborates. Nat. Protoc. 10, 1423–1432 (2015).
Wängler, B. et al. Protein labeling with the labeling precursor [18F]SiFa-SH for positron emission tomography. Nat. Protoc. 7, 1964–1969 (2012).
McBride, W. J., D’Souza, C. A., Sharkey, R. M. & Goldenberg, D. M. The radiolabeling of proteins by the [18F]AlF method. Appl. Radiat. Isot. 70, 200–204 (2012).
Cleeren, F. et al. New chelators for low temperature Al18F-labeling of biomolecules. Bioconjug. Chem. 27, 790–798 (2016).
McBride, W. J., Sharkey, R. M. & Goldenberg, D. M. Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res. 3, 36–47 (2013).
McBride, W. J. et al. A novel method of 18F radiolabeling for PET. J. Nucl. Med. 50, 991–998 (2009).
Wang, W., Liu, Z. & Li, Z. One-step 18F labeling of non-peptidic bivalent integrin α. Bioconjug. Chem. 26, 24–28 (2015).
Kiesewetter, D. O. et al. Evaluation of an [18F]AlF-NOTA analog of exendin-4 for imaging of GLP-1 receptor in insulinoma. Theranostics 2, 999–1009 (2012).
Da Pieve, C. et al. Efficient [18F]AlF radiolabeling of ZHER3:8698 affibody molecule for imaging of HER3 positive tumors. Bioconjug. Chem. 27, 1839–1849 (2016).
Kumar, K. & Ghosh, A. 18F-AlF labeled peptide and protein conjugates as Positron Emission Tomography imaging pharmaceuticals. Bioconjug. Chem. 29, 953–975 (2018).
Wan, W. et al. One-step 18F labeling of non-peptidic bivalent integrin α. Bioconjug. Chem. 26, 24–28 (2015).
Jadvar, H., Desai, B. & Conti, P. S. Sodium 18F-fluoride PET/CT of bone, joint, and other disorders. Semin. Nucl. Med. 45, 58–65 (2015).
Chatalic, K. L. S. et al. Preclinical comparison of Al18F- and 68Ga-labeled gastrin-releasing peptide receptor antagonists for PET imaging of prostate cancer. J. Nucl. Med. 55, 2050–2056 (2014).
Heskamp, S. et al. Imaging of human epidermal growth factor receptor type 2 expression with 18F-labeled affibody molecule ZHER2:2395 in a mouse model for ovarian cancer. J. Nucl. Med. 53, 146–153 (2012).
Cleeren, F. et al. Al18F-labeling of heat-sensitive biomolecules for positron emission tomography imaging. Theranostics 7, 2924–2939 (2017).
Brom, M. et al. Non-invasive quantification of the beta cell mass by SPECT with ¹¹¹In-labelled exendin. Diabetologia 57, 950–959 (2014).
Laverman, P. et al. A novel facile method of labeling octreotide with 18F-fluorine. J. Nucl. Med. 51, 454–461 (2010).
Sandström, M. et al. Biodistribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J. Nucl. Med. 57, 867–871 (2016).
Goldstein, R. et al. Development of the designed ankyrin repeat protein (DARPin) G3 for HER2 molecular imaging. Eur. J. Nucl. Med. Mol. Imaging 42, 288–301 (2015).
Garousi, J. et al. Influence of the N-terminal composition on targeting properties of radiometal-labeled anti-HER2 scaffold protein ADAPT6. Bioconjug. Chem. 27, 2678–2688 (2016).
Chakravarty, R. et al. Matching the decay half-life with the biological half-life: ImmunoPET imaging with 44Sc-labeled cetuximab Fab fragment. Bioconjug. Chem. 25, 2197–2204 (2014).
Sharma, S. K. et al. Synthesis and pre-clinical evaluation of an 18F-labeled single-chain antibody fragment for PET imaging of epithelial ovarian cancer. Am. J. Nucl. Med. Mol. Imaging 6, 185–198 (2016).
De Vos, J., Devoogdt, N., Lahoutte, T. & Muyldermans, S. Camelid single-domain antibody-fragment engineering for (pre)clinical in vivo molecular imaging applications: adjusting the bullet to its target. Expert Opin. Biol. Ther. 13, 1149–1160 (2013).
Pandit-Taskar, N. et al. First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J. Nucl. Med. 57, 1858–1864 (2016).
Bedford, R. et al. Alternative reagents to antibodies in imaging applications. Biophys. Rev. 9, 299–308 (2017).
Wu, A. M. Engineered antibodies for molecular imaging of cancer. Methods 65, 139–147 (2014).
De Meyer, T., Muyldermans, S. & Depicker, A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 32, 263–270 (2014).
D’Huyvetter, M. et al. Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics 4, 708–720 (2014).
Wen, Y. et al. Structural evaluation of a nanobody targeting complement receptor Vsig4 and its cross reactivity. Immunobiology 222, 807–813 (2017).
Massa, S., Xavier, C., Muyldermans, S. & Devoogdt, N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opin. Drug Deliv. 13, 1149–1163 (2016).
Adumeau, P., Sharma, S. K., Brent, C. & Zeglis, B. M. Site-specifically labeled immunoconjugates for molecular imaging—part 1: cysteine residues and glycans. Mol. Imaging Biol. 18, 1–17 (2016).
Adumeau, P., Sharma, S. K., Brent, C. & Zeglis, B. M. Site-specifically labeled immunoconjugates for molecular imaging—part 2: peptide tags and unnatural amino acids. Mol. Imaging Biol. 18, 153–165 (2016).
Kramer-Marek, G. et al. [18F]FBEM-ZHER2:342–affibody molecule—a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 35, 1008–1018 (2008).
Füchtner, F. et al. Factors affecting the specific activity of [18F]fluoride from a [18O]water target. Nuklearmedizin 47, 116–119 (2008).
Acknowledgements
The authors thank J. Cleynhens, J. Cornelis, I. Sannen, J. Peetroons, S. Celen, H.-J. Verhaegen, and P. Haspeslagh from the Laboratory for Radiopharmaceutical Research (University of Leuven); J. De Jonge from the In vivo Cellular and Molecular Imaging Center (Vrije Universiteit Brussel); and S. Muyldermans from the Cellular and Molecular Imaging Laboratory (Vrije Universiteit Brussel). This research received support from IWT Flanders (SBO 130065 MIRIAD, G.B., N.D.; SBO S000218N MATATUM, N.D.), the EU (H2020-MSCA-ITN PET3D, C.X., J.B., N.D.), and the FWO (G0D8817N, G.B.; G066615N, C.X., N.D.). F.C. is a Postdoctoral Fellow of FWO (12R3119N).
Author information
Authors and Affiliations
Contributions
F.C., J.L., N.D., C.X., and G.B. designed the research. J.L. synthesized the RESCA ligands. F.C., T.T., and J.B. performed the experimental work. F.C., T.T., and G.B. wrote the manuscript. G.B. is the corresponding author.
Corresponding author
Ethics declarations
Competing interests
A patent application related to this work has been filed (WO/2016/065435; F.C., J.L., G.B.). The RESCA chelator will be made commercially available from Chematech in the near future. F.C., J.L., and G.B. will receive a part of the profit. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Cleeren, F. et al. Theranostics 7, 2924–2939 (2017) http://www.thno.org/v07p2924.htm
Supplementary information
Rights and permissions
About this article
Cite this article
Cleeren, F., Lecina, J., Bridoux, J. et al. Direct fluorine-18 labeling of heat-sensitive biomolecules for positron emission tomography imaging using the Al18F-RESCA method. Nat Protoc 13, 2330–2347 (2018). https://doi.org/10.1038/s41596-018-0040-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0040-7
This article is cited by
-
ImmunoPET/CT imaging of clear cell renal cell carcinoma with [18F]RCCB6: a first-in-human study
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
High in-vivo stability in preclinical and first-in-human experiments with [18F]AlF-RESCA-MIRC213: a 18F-labeled nanobody as PET radiotracer for diagnosis of HER2-positive cancers
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Comparison of renal clearance of [18F]AlF-RESCA-HER2-BCH and [18F]AlF-NOTA-HER2-BCH in mice and breast cancer patients
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Aluminum fluoride-18-labelled indocyanine green as a potential PET imaging agent for hepatic function reserve
Journal of Radioanalytical and Nuclear Chemistry (2022)
-
Automated GMP compliant production of [18F]AlF-NOTA-octreotide
EJNMMI Radiopharmacy and Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.