Abstract
The anticodon modifications of transfer RNAs (tRNAs) finetune the codon recognition on the ribosome for accurate translation. Bacteria and archaea utilize the modified cytidines, lysidine (L) and agmatidine (agm2C), respectively, in the anticodon of tRNAIle to decipher AUA codon. L and agm2C contain long side chains with polar termini, but their functions remain elusive. Here we report the cryogenic electron microscopy structures of tRNAsIle recognizing the AUA codon on the ribosome. Both modifications interact with the third adenine of the codon via a unique C–A geometry. The side chains extend toward 3′ direction of the mRNA, and the polar termini form hydrogen bonds with 2′-OH of the residue 3′-adjacent to the AUA codon. Biochemical analyses demonstrated that AUA decoding is facilitated by the additional interaction between the polar termini of the modified cytidines and 2′-OH of the fourth mRNA residue. We also visualized cyclic N6-threonylcarbamoyladenosine (ct6A), another tRNA modification, and revealed a molecular basis how ct6A contributes to efficient decoding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Publicly available datasets from Protein Data Bank (7K00, 4V8N and 4V5R) were used for atomic model building and comparison. Cryo-EM maps and atomic coordinates of the reported structures were deposited in Electron Microscopy Data Bank (EMDB) and Protein Data Bank, respectively, with the following accession codes: EMD-35001 and 8HSP (EctRNAIle2 with t6A37); EMD-35022 and 8HU1 (EctRNAIle2 with ct6A37); EMD-35020 and 8HTZ (HmtRNAIle2). Source data are provided with this paper.
References
Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 22, 375–392 (2021).
Bjork, G. R. Biosynthesis and function of modified nucleosides. in tRNA: Structure, Biosynthesis, and Function (eds. Dieter Söll, D. & RajBhandary, U. L.) 165–205 (American Society for Microbiology, 1995).
Yokoyama, S. & Nishimura, S. Modified nucleosides and codon recognition. in tRNA: Structure, Biosynthesis, and Function (eds. Söll, D. & RajBhandary, U. L.) 207–223 (American Society for Microbiology, 1995).
Suzuki, T. in Fine-Tuning of RNA Functions by Modification and Editing Vol. 12 (ed. Grosjean, H.) 23–69 (Springer, 2005).
El Yacoubi, B., Bailly, M. & de Crecy-Lagard, V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 46, 69–95 (2012).
Suzuki, T. & Nagao, A. Genetic code and its variations. eLS 2, 147–157 (2021).
Suzuki, T. & Numata, T. Convergent evolution of AUA decoding in bacteria and archaea. RNA Biol. 11, 1586–1596 (2014).
Muramatsu, T. et al. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J. Biol. Chem. 263, 9261–9267 (1988).
Muramatsu, T. et al. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336, 179–181 (1988).
Soma, A. et al. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell 12, 689–698 (2003).
Suzuki, T. & Miyauchi, K. Discovery and characterization of tRNAIle lysidine synthetase (TilS). FEBS Lett. 584, 272–277 (2010).
Ikeuchi, Y. et al. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 6, 277–282 (2010).
Nakanishi, K. et al. Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase. Nature 461, 1144–1148 (2009).
Osawa, T. et al. Structural basis of tRNA agmatinylation essential for AUA codon decoding. Nat. Struct. Mol. Biol. 18, 1275–1280 (2011).
Terasaka, N., Kimura, S., Osawa, T., Numata, T. & Suzuki, T. Biogenesis of 2-agmatinylcytidine catalyzed by the dual protein and RNA kinase TiaS. Nat. Struct. Mol. Biol. 18, 1268–1274 (2011).
Voorhees, R. M. et al. The structural basis for specific decoding of AUA by isoleucine tRNA on the ribosome. Nat. Struct. Mol. Biol. 20, 641–643 (2013).
Schmeing, T. M., Voorhees, R. M., Kelley, A. C. & Ramakrishnan, V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat. Struct. Mol. Biol. 18, 432–436 (2011).
Hirsh, D. Tryptophan tRNA of Escherichia coli. Nature 228, 57 (1970).
Schweizer, M. P., Chheda, G. B., Baczynskyj, L. & Hall, R. H. Aminoacyl nucleosides. VII. N-(Purin-6-ylcarbamoyl)threonine. A new component of transfer ribonucleic acid. Biochemistry 8, 3283–3289 (1969).
Thiaville, P. C., Iwata-Reuyl, D. & de Crecy-Lagard, V. Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t6A), a universal modification of tRNA. RNA Biol. 11, 1529–1539 (2014).
Niimi, T. et al. Recognition of the anticodon loop of tRNAIle1 by isoleucyl-tRNA synthetase from Escherichia coli. Nucleosides Nucleotides 13, 1231–1237 (1994).
Yarian, C. et al. Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem. 277, 16391–16395 (2002).
Phelps, S. S., Malkiewicz, A., Agris, P. F. & Joseph, S. Modified nucleotides in tRNA(Lys) and tRNA(Val) are important for translocation. J. Mol. Biol. 338, 439–444 (2004).
El Yacoubi, B. et al. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 30, 882–893 (2011).
Lin, C. A., Ellis, S. R. & True, H. L. The Sua5 protein is essential for normal translational regulation in yeast. Mol. Cell. Biol. 30, 354–363 (2010).
Rozov, A. et al. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 7, 10457 (2016).
Murphy, F. V., Ramakrishnan, V., Malkiewicz, A. & Agris, P. F. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 11, 1186–1191 (2004).
Stuart, J. W. et al. Functional anticodon architecture of human tRNALys3 includes disruption of intraloop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry 39, 13396–13404 (2000).
Miyauchi, K., Kimura, S. & Suzuki, T. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat. Chem. Biol. 9, 105–111 (2013).
Matuszewski, M. et al. A hydantoin isoform of cyclic N6-threonylcarbamoyladenosine (ct6A) is present in tRNAs. Nucleic Acids Res. 45, 2137–2149 (2017).
Klassen, R. et al. tRNA anticodon loop modifications ensure protein homeostasis and cell morphogenesis in yeast. Nucleic Acids Res. 44, 10946–10959 (2016).
Bernier, C. R., Petrov, A. S., Kovacs, N. A., Penev, P. I. & Williams, L. D. Translation: the universal structural core of life. Mol. Biol. Evol. 35, 2065–2076 (2018).
Bai, X. C., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. Sampling the conformational space of the catalytic subunit of human gamma-secretase. eLife 4, e11182 (2015).
Ogle, J. M. et al. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292, 897–902 (2001).
Westhof, E., Watson, Z. L., Zirbel, C. L. & Cate, J. H. D. Anionic G*U pairs in bacterial ribosomal rRNAs. RNA 29, 1069–1076 (2023).
Jenner, L., Demeshkina, N., Yusupova, G. & Yusupov, M. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat. Struct. Mol. Biol. 17, 1072–1078 (2010).
Selmer, M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006).
Li, S., Olson, W. K. & Lu, X. J. Web 3DNA 2.0 for the analysis, visualization, and modeling of 3D nucleic acid structures. Nucleic Acids Res. 47, W26–W34 (2019).
Kurata, S. et al. Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U.G wobble pairing during decoding. J. Biol. Chem. 283, 18801–18811 (2008).
Murphy, F. V. T. & Ramakrishnan, V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat. Struct. Mol. Biol. 11, 1251–1252 (2004).
Cantara, W. A., Murphy, F. V. T., Demirci, H. & Agris, P. F. Expanded use of sense codons is regulated by modified cytidines in tRNA. Proc. Natl Acad. Sci. USA 110, 10964–10969 (2013).
Vendeix, F. A. et al. Human tRNA(Lys3)(UUU) is pre-structured by natural modifications for cognate and wobble codon binding through keto-enol tautomerism. J. Mol. Biol. 416, 467–485 (2012).
Fernandez, I. S. et al. Unusual base pairing during the decoding of a stop codon by the ribosome. Nature 500, 107–110 (2013).
Weixlbaumer, A. et al. Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat. Struct. Mol. Biol. 14, 498–502 (2007).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Stuart, J. W., Koshlap, K. M., Guenther, R. & Agris, P. F. Naturally-occurring modification restricts the anticodon domain conformational space of tRNA(Phe). J. Mol. Biol. 334, 901–918 (2003).
Miyauchi, K., Ohara, T. & Suzuki, T. Automated parallel isolation of multiple species of non-coding RNAs by the reciprocal circulating chromatography method. Nucleic Acids Res. 35, e24 (2007).
Ishiguro, K., Arai, T. & Suzuki, T. Depletion of S-adenosylmethionine impacts on ribosome biogenesis through hypomodification of a single rRNA methylation. Nucleic Acids Res. 47, 4226–4239 (2019).
Ohira, T. et al. Reversible RNA phosphorylation stabilizes tRNA for cellular thermotolerance. Nature 605, 372–379 (2022).
Takakura, M., Ishiguro, K., Akichika, S., Miyauchi, K. & Suzuki, T. Biogenesis and functions of aminocarboxypropyluridine in tRNA. Nat. Commun. 10, 5542 (2019).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Watson, Z. L. et al. Structure of the bacterial ribosome at 2 Å resolution. eLife 9, e60482 (2020).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Lu, X. J., Bussemaker, H. J. & Olson, W. K. DSSR: an integrated software tool for dissecting the spatial structure of RNA. Nucleic Acids Res. 43, e142 (2015).
Ogle, J. M., Murphy, F. V., Tarry, M. J. & Ramakrishnan, V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell 111, 721–732 (2002).
Acknowledgements
We are grateful to the members of the Suzuki laboratory, in particular, Y. Sakaguchi, for technical support and insightful discussion. Special thanks are due to M. Gagnon and M. Rybak (UTMB) for sharing unpublished data with us and productive discussion. All cryo-EM data in this study were collected at the cryo-EM facility of the RIKEN Center for Biosystems Dynamics Research (Yokohama). We thank T. Uchikubo-Kamo and R. Akasaka for their help with cryo-EM data collection and analysis. Radioisotope experiments were carried out with the support of the Isotope Science Center, Univ. of Tokyo. This work was supported by a grant in aid for scientific research from MEXT and JSPS (26113003, 26220205, and 18H05272 to T.S.; 26116003 and 25660053 to A.N.; 18K05430 to K.M.; 20J00947 to K.I.; 23KJ0409 to N.A.), AMED (JP21am0101115 to T.Y.; JP223fa627001 to T.S.; JP21am0101082 to M.S.), RIKEN (Pioneering project ‘Biology of Intracellular Environments’ and BDR Structural Cell Biology Project to M.S.) and JST (ERATO, JPMJER2002 to T.S.).
Author information
Authors and Affiliations
Contributions
N.A. prepared all materials, performed biochemical experiments assisted by A.N. and K.M. and conducted cryo-EM analyses supported by K.I., T.Y. and M.S. N.A. and T.S. wrote the manuscript. All authors discussed the results. T.S. designed and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Sara Osman was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mass spectrometric characterization of the native tRNAs used in this study.
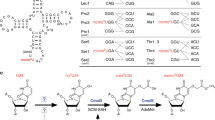
(a) Secondary structures of E. coli (left panel) and H. marismortui (right panel) tRNAIle2 with post-transcriptional modifications. Abbreviations are as follows: s4U, 4-thiouridine; Gm, 2’-O-methylguanosine; D, dihydrouridine; L, lysidine; t6A, N6-threonylcarbamoyladenosine; Ψ, pseudouridine; m7G, 7-methylguanosine; acp3U, 3-(3-amino-3-carboxypropyl)uridine; T, 5-methyluridine; G+, archaeosine; m2,2G, N2, N2-dimethylguanosine; agm2C, agmatidine; m5C, 5-methylcytidine; m1Ψ, 1-methylpseudouridine; Cm, 2’-O-methylcytidine; and m1I, 1-methylinosine. The sequence colored in red corresponds to the RNase T1 fragment containing the anticodon region. (b) PAGE analysis of tRNAIle2 isolated by RCC. Total RNAs are used as size markers. A band of impurity is asterisked. Isolation and electrophoresis of tRNA were repeated at least three times, yielding similar results. The representative gel image is shown. (c) Mass spectrometric analyses of tRNAIle2 isolated from the E. coli ΔtcdA strain (left panels) and H. marismortui (right panels). Extracted-ion chromatograms (XICs) of C34-containing fragments (top panels) and L34- or agm2C-containing fragments (bottom panels) of tRNAIle2 digested by RNase T1. The sequences of the detected fragments with m/z values and charge states are indicated on the right.
Extended Data Fig. 2 In vitro reconstitution of ct6A37 in E. coli tRNAIle2.
(a) Schematic depiction of ct6A formation catalyzed by TcdA in the presence of ATP. (b) Mass spectrometric analyses of E. coli tRNAIle2 before (left panels) and after (right panels) ct6A reconstitution. XICs of t6A37-containing fragments (upper panels) and ct6A37-containing fragments (lower panels) of E. coli tRNAIle2 digested by RNase A and treated with BAP. The sequences of the detected fragments with m/z values and charge states are indicated on the right.
Extended Data Fig. 3 Structural analyses of 70 S ribosome complexed with A- and P-site tRNAs.
(a) Image processing of the ribosome complexes with E. coli or H. marismortui tRNAIle2 at the A-site. Auto-picked particle images were subjected to 2D classification in order to remove low quality particles and auto-refinement. After 3D classification, the subclasses of 70 S ribosomes with P-site occupancy were pooled for 3D refinement; this pool included all particles potentially bound by A-site tRNAIle2. Particles with A-site density were extracted by focused classification using an A-site mask and used to generate the final map. The overall cryo-EM maps are shown and colored according to the local resolution. (b, c) Fourier shell correlation (FSC) curves of the complexes (b) and models vs. cryo-EM maps (c). (d) Isolated densities of tRNA and mRNA colored according to the local resolution. From left to right, EctRNAIle2 + t6A, EctRNAIle2+ct6A, HmtRNAIle2.
Extended Data Fig. 4 Cryo-EM structure of H. marismortui tRNAIle2.
(a) Atomic model of the E. coli 70 S ribosome (23 S; light green, 16 S; olive, r-proteins; yellow) bound with E. coli tRNAGlu at the P-site (magenta) and H. marismortui tRNAIle2 at the A-site (orange). The molecular surface is displayed on each tRNA model. (b) Codon-anticodon duplex of H. marismortui tRNAIle2 (orange) and the AUA codon (gray) in the decoding center of 16 S rRNA (olive). A1913 (light green) in Helix 69 of 23 S rRNA and S12 (yellow) are displayed. (c) The architecture of the decoding center around L34 (left panel) or agm2C34 (right panel). The backbone of mRNA third residue is coordinated with a potassium ion bound to C518 and G530 of 16 S rRNA, and Pro45 of S12.
Extended Data Fig. 5 Codon-anticodon interactions at the P-site.
The codon-anticodon duplex of E. coli tRNAGlu (purple) and the GAG codon (gray) at the P-site (left panel) in the complex with E. coli tRNAIle2(+t6A). Base pairing geometry of mnm5s2U34 recognizing G3 of the GAG codon. Post-processed maps (contoured at level 0.02) are superimposed on the model structure. The geometry of the minor groove-shifted wobble base pair is identical to that at the A-site described previously26. The density of the methylaminomethyl (mnm) group at the C5 atom is visible at this contour level.
Extended Data Fig. 6 Comparison of base pairing geometries between C34-G3 (gray), L34-A3 (blue), and agm2C-A3 (orange).
When C*34-A3 pairs and C34-G3 canonical Watson-Crick pair (PDB ID: 4V5R) are superimposed by the mRNA base, the cytosine base of C*34 is displaced to its minor groove by 2.7–2.8 Å.
Extended Data Fig. 7 Fitting of the C*34 side chain into the cleft formed by rRNA and mRNA.
Solvent-excluded surface models showing the structural complementarity of the long side chains of L34 (left panel) and agm2C (right panel), and the cleft formed by rRNA residues and the mRNA strand. A large area of van der Waals contacts between C*34 and rRNA residues and the mRNA is clearly visible, especially in the agm2C model.
Extended Data Fig. 8 Conformational restriction of the hydantoin ring of ct6A37.
(a) Ab initio conformational energies in the ct6A nucleoside for the rigid scan over the dihedral angle η (N1-C6-N6-C13)30. \(\eta =\)21.7° (suggested in our model) is indicated by red line. Twisted rotamers with different η showing the minimum energies are shown by blue line. (b) Atomic model of ct6A37 and the neighboring residues based on our cryo-EM map (\(\eta =\)21.7°). (c-f) Simulated atomic models of ct6A37 with different η showing the minimum energies suggested by quantum calculations30 in (a). ct6A37 was modeled with the following dihedral angles η of the hydantoin ring and the adenine base; (c) \(\eta =\)67.3°; (d) \(\eta =\)127.3°; (e) \(\eta =\)-52.7° (consistent with the single crystal structure30); (f) \(\eta =\)-122.7°. In these models, the hydantoin ring shows an apparent steric clash with neighboring nucleotides in the codon-anticodon helix.
Extended Data Fig. 9 Comparison of previous and current models of agm2C34 recognizing A3 of the AUA codon.
The C-A geometry is consistent between the previous model (yellow, PDB ID: 4V8N) and the current model (orange). However, the orientation of the agmatinyl moiety is markedly different. In our structure, the clear density of agm2C34 enables to fully model its long side chain with a terminal guanidino group.
Extended Data Fig. 10 Alternative structural model of L34 recognizing the fourth mRNA residue.
(a) Alternative interactions of L34 recognizing the AUA codon at the A-site. The terminal amino group of L34 has a potential to form an H-bond with 2’-OH of the fourth mRNA residue (N4) considering 2’-F substitution maintained AUA decoding efficiency. (b) Simulated atomic model of L34 rotamer in which the amino group of L34 serves as a hydrogen donor.
Supplementary information
Source data
Source Data Fig. 3
Statistical source data for Fig. 3c,e.
Source Data Extended Data Fig. 1
Unprocessed gel image.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akiyama, N., Ishiguro, K., Yokoyama, T. et al. Structural insights into the decoding capability of isoleucine tRNAs with lysidine and agmatidine. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01238-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01238-1