Abstract
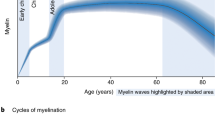
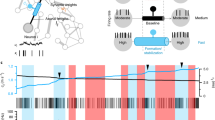
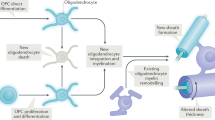
Myelin, which is produced by oligodendrocytes, insulates axons to facilitate rapid and efficient action potential propagation in the central nervous system. Traditionally viewed as a stable structure, myelin is now known to undergo dynamic modulation throughout life. This Review examines these dynamics, focusing on two key aspects: (1) the turnover of myelin, involving not only the renewal of constituents but the continuous wholesale replacement of myelin membranes; and (2) the structural remodeling of pre-existing, mature myelin, a newly discovered form of neural plasticity that can be stimulated by external factors, including neuronal activity, behavioral experience and injury. We explore the mechanisms regulating these dynamics and speculate that myelin remodeling could be driven by an asymmetry in myelin turnover or reactivation of pathways involved in myelin formation. Finally, we outline how myelin remodeling could have profound impacts on neural function, serving as an integral component of behavioral adaptation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Young, K. M. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Tripathi, R. B. et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 21, 316–323 (2017).
Hill, R. A., Li, A. M. & Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695 (2018).
Wang, F. et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486 (2020).
Yeung, M. S. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Price, J. C., Guan, S., Burlingame, A., Prusiner, S. B. & Ghaemmaghami, S. Analysis of proteome dynamics in the mouse brain. Proc. Natl Acad. Sci. USA 107, 14508–14513 (2010). Landmark study finding that myelin proteins are some of the longest lived in the body.
Fornasiero, E. F. et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 9, 4230 (2018).
Goh, B., Kim, J., Seo, S. & Kim, T.-Y. High-throughput measurement of lipid turnover rates using partial metabolic heavy water labeling. Anal. Chem. 90, 6509–6518 (2018).
Poitelon, Y., Kopec, A. M. & Belin, S. Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells 9, 812 (2020).
Ando, S., Tanaka, Y., Toyoda, Y. & Kon, K. Turnover of myelin lipids in aging brain. Neurochem. Res. 28, 5–13 (2003). Demonstration of variable lifetimes of myelin lipids.
Dustin, E. et al. Compromised myelin and axonal molecular organization following adult-onset sulfatide depletion. Biomedicines 11, 1431 (2023).
Snaidero, N. et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290 (2014).
Simons, M., Krämer, E.-M., Thiele, C., Stoffel, W. & Trotter, J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J. Cell Biol. 151, 143–154 (2000).
Pralle, A., Keller, P., Florin, E.-L., Simons, K. & Hörber, J. K. H. Sphingolipid–cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008 (2000).
Kato, D. et al. Regulation of lipid synthesis in myelin modulates neural activity and is required for motor learning. Glia 71, 2591–2608 (2023).
Zhou, X. et al. Mature myelin maintenance requires Qki to coactivate PPARβ–RXRα-mediated lipid metabolism. J. Clin. Invest. 130, 2220–2236 (2020).
Stadelmann, C., Timmler, S., Barrantes-Freer, A. & Simons, M. Myelin in the central nervous system: structure, function, and pathology. Physiol. Rev. 99, 1381–1431 (2019).
Toyama, B. H. et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982 (2013).
Savas, J. N., Toyama, B. H., Xu, T., Yates, J. R. & Hetzer, M. W. Extremely long-lived nuclear pore proteins in the rat brain. Science 335, 942 (2012).
Lüders, K. A. et al. Maintenance of high proteolipid protein level in adult central nervous system myelin is required to preserve the integrity of myelin and axons. Glia 67, 634–649 (2019).
Meschkat, M. et al. White matter integrity in mice requires continuous myelin synthesis at the inner tongue. Nat. Commun. 13, 1163 (2022). Landmark observations on the continuous turnover of entire myelin membranes.
Lin, Y. et al. Impaired eukaryotic translation initiation factor 2B activity specifically in oligodendrocytes reproduces the pathology of vanishing white matter disease in mice. J. Neurosci. 34, 12182–12191 (2014).
Ishii, A., Furusho, M., Dupree, J. L. & Bansal, R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J. Neurosci. 34, 16031–16045 (2014).
Aber, E. R. et al. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 41, 111480 (2022).
Ktena, N. et al. Autophagic degradation of CNS myelin maintains axon integrity. Cell Stress 6, 93–107 (2022).
Readhead, C. et al. Expression of a myelin basic protein gene in transgenic shiverer mice: correction of the dysmyelinating phenotype. Cell 48, 703–712 (1987).
Hildebrand, C., Remahl, S., Persson, H. & Bjartmar, C. Myelinated nerve fibres in the CNS. Prog. Neurobiol. 40, 319–384 (1993).
Djannatian, M. et al. Myelination generates aberrant ultrastructure that is resolved by microglia. J. Cell Biol. 222, e202204010 (2023).
Krämer‐Albers, E.-M. et al. Oligodendrocytes secrete exosomes containing major myelin and stress‐protective proteins: trophic support for axons? Proteomics Clin. Appl. 1, 1446–1461 (2007).
Mills, E. A. et al. Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc. Natl Acad. Sci. USA 112, 10509–10514 (2015).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Chapman, T. W., Olveda, G. E., Bame, X., Pereira, E. & Hill, R. A. Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice. Nat. Neurosci. 26, 555–569 (2023).
Sturrock, R. R. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420 (1980).
McNamara, N. B. et al. Microglia regulate central nervous system myelin growth and integrity. Nature 613, 120–129 (2023).
Peters, A., Sethares, C. & Killiany, R. J. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J. Comp. Neurol. 435, 241–248 (2001).
Furusho, M., Dupree, J. L., Nave, K.-A. & Bansal, R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci. 32, 6631–6641 (2012).
Blakemore, W. F. Pattern of remyelination in the CNS. Nature 249, 577–578 (1974).
Shepherd, M. N., Pomicter, A. D., Velazco, C. S., Henderson, S. C. & Dupree, J. L. Paranodal reorganization results in the depletion of transverse bands in the aged central nervous system. Neurobiol. Aging 33, 203.e13–203.e24 (2012).
Geraghty, A. C. et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265 (2019).
Mitew, S. et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9, 306 (2018).
Knowles, J. K. et al. Maladaptive myelination promotes generalized epilepsy progression. Nat. Neurosci. 25, 596–606 (2022).
Liu, J. et al. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 36, 957–962 (2016).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Bonnefil, V. et al. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. eLife 8, e40855 (2019).
Nicholson, M. et al. Remodelling of myelinated axons and oligodendrocyte differentiation is stimulated by environmental enrichment in the young adult brain. Eur. J. Neurosci. 56, 6099–6114 (2022).
Sinclair, J. L. et al. Sound-evoked activity influences myelination of brainstem axons in the trapezoid body. J. Neurosci. 37, 8239–8255 (2017).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Makinodan, M., Rosen, K. M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Yang, Y. et al. Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of Wnt signaling. Cereb. Cortex 27, 2871–2884 (2017).
Osanai, Y. et al. Dark rearing in the visual critical period causes structural changes in myelinated axons in the adult mouse visual pathway. Neurochem. Res. 47, 2815–2825 (2022).
Jeffries, M. A. et al. ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J. Neurosci. 36, 9186–9200 (2016).
Snaidero, N. et al. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat. Commun. 11, 4901 (2020).
Lebrun-Julien, F. et al. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J. Neurosci. 34, 8432–8448 (2014).
Ishii, A., Furusho, M., Dupree, J. L. & Bansal, R. Strength of ERK1/2 MAPK activation determines its effect on myelin and axonal integrity in the adult CNS. J. Neurosci. 36, 6471–6487 (2016).
Goebbels, S. et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 30, 8953–8964 (2010). This study demonstrated that mature sheaths can reinitiate rapid radial growth.
Furusho, M., Ishii, A. & Bansal, R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2–MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci. 37, 2931–2946 (2017).
Asadollahi, E. et al. Myelin lipids as nervous system energy reserves. Preprint at bioRxiv https://doi.org/10.1101/2022.02.24.481621 (2022). This study found that β-oxidation of myelin lipids is used to support neuronal energetics.
Cohen, C. H. C. et al. Saltatory conduction along myelinated axons involves a periaxonal nanocircuit. Cell 180, 311–322 (2020).
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Cullen, C. L. et al. Periaxonal and nodal plasticities modulate action potential conduction in the adult mouse brain. Cell Rep. 34, 108641 (2021).
Marcus, J., Dupree, J. L. & Popko, B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo–glial interactions. J. Cell Biol. 156, 567–577 (2002).
Li, C. et al. Myelination in the absence of myelin-associated glycoprotein. Nature 369, 747–750 (1994).
Montag, D. et al. Mice deficient for the glycoprotein show subtle abnormalities in myelin. Neuron 13, 229–246 (1994).
Pronker, M. F. et al. Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun. 7, 13584 (2016).
Menichella, D. M., Goodenough, D. A., Sirkowski, E., Scherer, S. S. & Paul, D. L. Connexins are critical for normal myelination in the CNS. J. Neurosci. 23, 5963–5973 (2003).
Menichella, D. M. et al. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J. Neurosci. 26, 10984–10991 (2006).
Larson, V. A. et al. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. eLife 7, e34829 (2018).
Schirmer, L. et al. Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. eLife 7, e36428 (2018).
Marshall-Phelps, K. L. H. et al. Neuronal activity disrupts myelinated axon integrity in the absence of NKCC1b. J. Cell Biol. 219, e201909022 (2020).
Auer, F., Vagionitis, S. & Czopka, T. Evidence for myelin sheath remodeling in the CNS revealed by in vivo imaging. Curr. Biol. 28, 549–559 (2018).
Bacmeister, C. M. et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 23, 819–831 (2020).
Yang, S. M., Michel, K., Jokhi, V., Nedivi, E. & Arlotta, P. Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370, eabd2109 (2020).
Bacmeister, C. M. et al. Motor learning drives dynamic patterns of intermittent myelination on learning-activated axons. Nat. Neurosci. 25, 1300–1313 (2022). This study found internode retraction along learning-activated axons.
Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J. & Bergles, D. E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706 (2018).
Lasiene, J., Matsui, A., Sawa, Y., Wong, F. & Horner, P. J. Age related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8, 201–213 (2009).
Mezydlo, A. et al. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron 111, 1748–1759 (2023).
Fu, Y., Sun, W., Shi, Y., Shi, R. & Cheng, J.-X. Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS ONE 4, e6705 (2009).
Huff, T. B. et al. Real-time CARS imaging reveals a calpain-dependent pathway for paranodal myelin retraction during high-frequency stimulation. PLoS ONE 6, e17176 (2011).
Hughes, A. N. & Appel, B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat. Commun. 10, 4125 (2019).
Elazar, N. et al. Axoglial adhesion by Cadm4 regulates CNS myelination. Neuron 101, 224–231 (2019).
Djannatian, M. et al. Two adhesive systems cooperatively regulate axon ensheathment and myelin growth in the CNS. Nat. Commun. 10, 4794 (2019).
Kearns, C. A., Ravanelli, A. M., Cooper, K. & Appel, B. Fbxw7 limits myelination by inhibiting mTOR signaling. J. Neurosci. 35, 14861–14871 (2015).
Fu, M.-M. M. et al. The Golgi outpost protein TPPP nucleates microtubules and is critical for myelination. Cell 179, 132–146 (2019).
Swire, M. et al. Oligodendrocyte HCN2 channels regulate myelin sheath length. J. Neurosci. 41, 7954–7964 (2021).
Etxeberria, A. et al. Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J. Neurosci. 36, 6937–6948 (2016).
Osanai, Y. et al. Length of myelin internodes of individual oligodendrocytes is controlled by microenvironment influenced by normal and input‐deprived axonal activities in sensory deprived mouse models. Glia 66, 2514–2525 (2018).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Koudelka, S. et al. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455 (2016).
Krasnow, A. M., Ford, M. C., Valdivia, L. E., Wilson, S. W. & Attwell, D. Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci. 21, 24–28 (2018).
Almeida, R. G. et al. Myelination induces axonal hotspots of synaptic vesicle fusion that promote sheath growth. Curr. Biol. 31, 3743–3754 (2021).
Wake, H. et al. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6, 7844 (2015).
Baraban, M., Koudelka, S. & Lyons, D. A. Ca2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci. 21, 19–23 (2018).
Iyer, M. et al. Oligodendrocyte calcium signaling promotes actin-dependent myelin sheath extension. Nat. Commun. 15, 265 (2024).
Battefeld, A., Popovic, M. A., de Vries, S. I. & Kole, M. H. P. High-frequency microdomain Ca2+ transients and waves during early myelin internode remodeling. Cell Rep. 26, 182–191 (2019).
Czopka, T., Ffrench-Constant, C. & Lyons, D. A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013).
Liu, P., Du, J. & He, C. Developmental pruning of early-stage myelin segments during CNS myelination in vivo. Cell Res. 23, 962–964 (2013).
Almeida, A. R. & Macklin, W. B. Early myelination involves the dynamic and repetitive ensheathment of axons which resolves through a low and consistent stabilization rate. eLife 12, e82111 (2023).
Neely, S. A. et al. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nat. Neurosci. 25, 415–420 (2022).
Duncan, I. D. et al. The adult oligodendrocyte can participate in remyelination. Proc. Natl Acad. Sci. USA 115, 11807–11816 (2018).
Yeung, M. S. Y. et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 (2019).
Swire, M., Kotelevtsev, Y., Webb, D. J., Lyons, D. A. & Ffrench-Constant, C. Endothelin signalling mediates experience-dependent myelination in the CNS. eLife 8, e49493 (2019).
Roy, K. et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl Acad. Sci. USA 104, 8131–8136 (2007).
Bechler, M. E., Byrne, L. & ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Câmara, J. et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J. Cell Biol. 185, 699–712 (2009).
Chong, S. Y. et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl Acad. Sci. USA 109, 1299–1304 (2012).
Harboe, M., Torvund‐Jensen, J., Kjaer‐Sorensen, K. & Laursen, L. S. Ephrin‐A1–EphA4 signaling negatively regulates myelination in the central nervous system. Glia 66, 934–950 (2018).
Rasband, M. N. & Peles, E. Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci. 22, 7–20 (2021).
Kuba, H., Oichi, Y. & Ohmori, H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature 465, 1075–1078 (2010).
Grubb, M. S. & Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074 (2010).
Dutta, D. J. et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl Acad. Sci. USA 115, 11832–11837 (2018).
Orthmann-Murphy, J. et al. Remyelination alters the pattern of myelin in the cerebral cortex. eLife 9, e56621 (2020).
Craner, M. J., Lo, A. C., Black, J. A. & Waxman, S. G. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 126, 1552–1561 (2003).
Coman, I. et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129, 3186–3195 (2006).
Vagionitis, S. et al. Clusters of neuronal neurofascin prefigure the position of a subset of nodes of Ranvier along individual central nervous system axons in vivo. Cell Rep. 38, 110366 (2022).
Young, R. G., Castelfranco, A. M. & Hartline, D. K. The ‘Lillie transition’: models of the onset of saltatory conduction in myelinating axons. J. Comput. Neurosci. 34, 533–546 (2013).
Moore, S. et al. A role of oligodendrocytes in information processing. Nat. Commun. 11, 5497 (2020).
Arancibia-Cárcamo, I. L. et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife 6, e23329 (2017).
Ford, M. C. et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun. 6, 8073 (2015).
Tomassy, G. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014).
Micheva, K. D., Kiraly, M., Perez, M. M. & Madison, D. V. Conduction velocity along the local axons of parvalbumin interneurons correlates with the degree of axonal myelination. Cereb. Cortex 31, 3374–3392 (2021).
Lang, E. J. & Rosenbluth, J. Role of myelination in the development of a uniform olivocerebellar conduction time. J. Neurophysiol. 89, 2259–2270 (2003).
Kato, D. et al. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210 (2020).
Steadman, P. E. et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164 (2020). This study identified a role for new oligodendrocytes in promoting neuronal synchrony.
Talidou, A., Frankland, P. W., Mabbott, D. & Lefebvre, J. Homeostatic coordination and up-regulation of neural activity by activity-dependent myelination. Nat. Comput. Sci. 2, 665–676 (2022).
Pajevic, S., Plenz, D., Basser, P. J. & Fields, R. D. Oligodendrocyte-mediated myelin plasticity and its role in neural synchronization. eLife 12, e81982 (2023).
Cordano, C. et al. Validating visual evoked potentials as a preclinical, quantitative biomarker for remyelination efficacy. Brain 145, 3943–3952 (2022).
Stedehouder, J., Brizee, D., Shpak, G. & Kushner, S. A. Activity-dependent myelination of parvalbumin interneurons mediated by axonal morphological plasticity. J. Neurosci. 38, 3631–3642 (2018).
Kerkoerle, T., van, Marik, S. A., Borgloh, S. M., zum, A. & Gilbert, C. D. Axonal plasticity associated with perceptual learning in adult macaque primary visual cortex. Proc. Natl Acad. Sci. USA 115, 10464–10469 (2018).
Johnson, C. M., Peckler, H., Tai, L.-H. & Wilbrecht, L. Rule learning enhances structural plasticity of long-range axons in frontal cortex. Nat. Commun. 7, 10785 (2016).
Hasegawa, R., Ebina, T., Tanaka, Y. R., Kobayashi, K. & Matsuzaki, M. Structural dynamics and stability of corticocortical and thalamocortical axon terminals during motor learning. PLoS ONE 15, e0234930 (2020).
Pan-Vazquez, A., Wefelmeyer, W., Sabater, V. G., Neves, G. & Burrone, J. Activity-dependent plasticity of axo-axonic synapses at the axon initial segment. Neuron 106, 265–276 (2020).
Stedehouder, J. et al. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb. Cortex 27, 5001–5013 (2017).
Stedehouder, J. et al. Local axonal morphology guides the topography of interneuron myelination in mouse and human neocortex. eLife 8, e48615 (2019).
Benamer, N., Vidal, M., Balia, M. & Angulo, M. C. Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat. Commun. 11, 5151 (2020).
Wang, K. C. et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002).
McGee, A. W., Yang, Y., Fischer, Q. S., Daw, N. W. & Strittmatter, S. M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226 (2005).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Meyer, N., Richter, N., Fan, Z., Siemonsmeier, G. & Pivneva, T. Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep. 22, 2383–2394 (2018).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Nave, K.-A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11, 275–283 (2010).
Rao-Ruiz, P., Yu, J., Kushner, S. A. & Josselyn, S. A. Neuronal competition: microcircuit mechanisms define the sparsity of the engram. Curr. Opin. Neurobiol. 54, 163–170 (2019).
Bollmann, Y. et al. Prominent in vivo influence of single interneurons in the developing barrel cortex. Nat. Neurosci. 26, 1555–1565 (2023).
Shenoy, K. V. & Kao, J. C. Measurement, manipulation and modeling of brain-wide neural population dynamics. Nat. Commun. 12, 633 (2021).
Colman, D. R., Kreibich, G., Frey, A. B. & Sabatini, D. D. Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol. 95, 598–608 (1982).
Zuchero, J. B. et al. CNS myelin wrapping is driven by actin disassembly. Dev. Cell 34, 152–167 (2015).
Nawaz, S. et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell 34, 139–151 (2015).
Bishop, G. H., Clare, M. H. & Landau, W. M. The relation of axon sheath thickness to fiber size in the central nervous system of vertebrates. Int. J. Neurosci. 2, 69–77 (1971).
Velumian, A. A., Samoilova, M. & Fehlings, M. G. Visualization of cytoplasmic diffusion within living myelin sheaths of CNS white matter axons using microinjection of the fluorescent dye Lucifer yellow. NeuroImage 56, 27–34 (2011).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Acknowledgements
We thank members of the Hughes laboratory for their helpful comments. Funding was provided by the National Multiple Sclerosis Society (FG-2208-40305) and the Department of Defense Congressionally Directed Medical Research Programs (MS220187) to L.A.O. Funding was provided by a University of Colorado Department of Cell and Developmental Biology pilot grant, the Whitehall Foundation, the National Multiple Sclerosis Society (RG-1701–26733) and the National Institute of Neurological Disorders and Stroke (NS115975, NS125230 and NS132859) to E.G.H.
Author information
Authors and Affiliations
Contributions
Conceptualization: L.A.O. and E.G.H. Original draft: L.A.O. Editing and review: L.A.O. and E.G.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Maria Cecilia Angulo, Klaus-Armin Nave, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osso, L.A., Hughes, E.G. Dynamics of mature myelin. Nat Neurosci (2024). https://doi.org/10.1038/s41593-024-01642-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41593-024-01642-2