Abstract
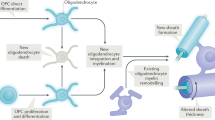
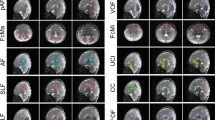
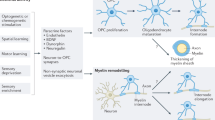
Myelin, a lipid membrane that wraps axons, enabling fast neurotransmission and metabolic support to axons, is conventionally thought of as a static structure that is set early in development. However, recent evidence indicates that in the central nervous system (CNS), myelination is a protracted and plastic process, ongoing throughout adulthood. Importantly, myelin is emerging as a potential modulator of neuronal networks, and evidence from human studies has highlighted myelin as a major player in shaping human behavior and learning. Here we review how myelin changes throughout life and with learning. We discuss potential mechanisms of myelination at different life stages, explore whether myelin plasticity provides the regenerative potential of the CNS white matter, and question whether changes in myelin may underlie neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nave, K. A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11, 275–283 (2010).
Alcami, P. & El Hady, A. Axonal computations. Front. Cell. Neurosci. 13, 413 (2019).
Pease-Raissi, S. E. & Chan, J. R. Building a (w)rapport between neurons and oligodendroglia: reciprocal interactions underlying adaptive myelination. Neuron 109, 1258–1273 (2021).
Chorghay, Z., Karadottir, R. T. & Ruthazer, E. S. White matter plasticity keeps the brain in tune: axons conduct while glia wrap. Front. Cell. Neurosci. 12, 428 (2018).
Norbom, L. B. et al. Maturation of cortical microstructure and cognitive development in childhood and adolescence: a T1w/T2w ratio MRI study. Hum. Brain Mapp. https://doi.org/10.1002/hbm.25149 (2020).
Grydeland, H. et al. Waves of maturation and senescence in micro-structural MRI markers of human cortical myelination over the life span. Cereb. Cortex 29, 1369–1381 (2019).
Reynolds, J. E., Grohs, M. N., Dewey, D. & Lebel, C. Global and regional white matter development in early childhood. Neuroimage 196, 49–58 (2019).
Westlye, L. T. et al. Life-span changes of the human brain white matter: diffusion tensor imaging and volumetry. Cereb. Cortex 20, 2055–2068 (2010).
Bernheimer, S. Über die Entwicklung und den Verlauf der Markfasern im Chiasma Nervorum Opticorum des Menschen (1889).
Westphal, A. Uber die Markscheidenbildung der Gehirnnerven des Menschen (1897).
Flechsig, P. Developmental (mye-logenetic) localisation of the cerebral cortex in the human subject. Lancet 2, 1027–1029 (1901).
Kaes, T. Die Grosshirnrinde des Menschen in ihren Massen und in inhrem Fasergehalt (Gustav Fischer, 1907). A detailed histological study providing clear evidence that human cortical myelination is a protracted process going well into the sixth or seventh decade.
Flechsig, P. E. Meine Myelogenetische Hirnlehre (Springer, 1927); https://doi.org/10.1007/978-3-662-32788-3. Summary of Flechsig’s pioneering histological studies providing clear evidence of human myelination during development and the notion of a link between myelination and circuit function.
Yakovlev, P. L. & Lecours, A. R. in Regional Development of the Brain in Early Life (ed. Minkowski, A.) 33–70 (Blackwell Scientific, 1967). An important paper detailing myelination in early human life providing evidence that myelination occurs in different stages through development and that brain areas myelinate at different times and rates. Provides evidence for myelination cycles.
Li, M. et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science https://doi.org/10.1126/science.aat7615 (2018).
Hill, R. A., Li, A. M. & Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695 (2018). Provides evidence that myelination is a protracted process in the mouse, and that myelin sheath lengths are plastic.
Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J. & Bergles, D. E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706 (2018). Provides evidence that myelination is a protracted process in the mouse, and that myelin sheath lengths are plastic.
Young, K. M. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Yang, S. M., Michel, K., Jokhi, V., Nedivi, E., Arlotta, P. Neuron-class-specific responses govern adaptive remodeling of myelination in the neocortex. Science https://doi.org/10.1126/science.abd2109 (2020). Longitudinal study indicating that existing myelin internodes change, dissappear and new ones appear, with experience in the mouse.
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). This paper provides in vivo evidence that direct changes in neuronal activity change myelin thickness and OPC proliferation and differentiation in the adult mouse. Provides important evidence for the notion of myelin plasticity.
Sinclair, J. L. et al. Sound-evoked activity influences myelination of brainstem axons in the trapezoid body. J. Neurosci. 37, 8239–8255 (2017).
Mitew, S. et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9, 306 (2018).
Miyata, S. et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 6, 23084 (2016). Cross-sectional study indicating that nodes change with experience in the mouse.
Cullen, C. L. et al. Periaxonal and nodal plasticities modulate action potential conduction in the adult mouse brain. Cell Rep. https://doi.org/10.1016/j.celrep.2020.108641 (2021).
Prins, N. D. & Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 11, 157–165 (2015).
Koshiyama, D. et al. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2,937 individuals. Mol. Psychiatry 25, 883–895 (2020).
Hoglinger, G. U. et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705 (2011).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Castelijns, B. et al. Hominin-specific regulatory elements selectively emerged in oligodendrocytes and are disrupted in autism patients. Nat. Commun. 11, 301 (2020).
Nagy, C. et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 23, 771–781 (2020).
Phan, B. N. et al. A myelin-related transcriptomic profile is shared by Pitt–Hopkins syndrome models and human autism spectrum disorder. Nat. Neurosci. 23, 375–385 (2020).
Zhao, B. et al. Common genetic variation influencing human white matter microstructure. Science https://doi.org/10.1126/science.abf3736 (2021).
Dean, D. C. 3rd et al. Characterizing longitudinal white matter development during early childhood. Brain Struct. Funct. 220, 1921–1933 (2015).
Flurkey, K., Currer, J. M. & Harrison, D. E. in The Mouse in Biomedical Research (eds Fox J. G. et al.) Ch. 20 (Academic Press, 2007).
Miller, D. J. et al. Prolonged myelination in human neocortical evolution. Proc. Natl Acad. Sci. USA 109, 16480–16485 (2012).
Gibson, K. R. Sequence of myelinization in the brain of Macaca mulatta. Dissertation, Univ. California, Berkeley (1970).
Tilney, F. & Casamajor, L. Myelinogeny as applied to the study of behavior. Arch. NeurPsych 12, 1–66 (1924).
Raz, N. in The Handbook of Aging and Cognition (eds Salthouse, T. A. & Criag, F. M.) 1–90 (Lawrence Erlbaum Associates, 2000).
Benes, F. M., Turtle, M., Khan, Y. & Farol, P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence and adulthood. Arch. Gen. Psychiatry 51, 477–484 (1994).
Olson, I. R., Von Der Heide, R. J., Alm, K. H. & Vyas, G. Development of the uncinate fasciculus: implications for theory and developmental disorders. Dev. Cogn. Neurosci. 14, 50–61 (2015).
Burgel, U. et al. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29, 1092–1105 (2006).
Skoff, R. P., Toland, D. & Nast, E. Pattern of myelination and distribution of neuroglial cells along the developing optic system of the rat and rabbit. J. Comp. Neurol. 191, 237–253 (1980).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014). This study changed the view of myelinated axons, providing clear evidence that axons can be intermittently myelinated.
Dangata, Y. Y., Findlater, G. S. & Kaufman, M. H. Postnatal development of the optic nerve in (C57BL × CBA)F1 hybrid mice: general changes in morphometric parameters. J. Anat. 189, 117–125 (1996).
Yeung, M. S. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Hughes, E. G., Kang, S. H., Fukaya, M. & Bergles, D. E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676 (2013).
Kamen, Y., Pivonkova, H., Evans, K. A. & Káradóttir, R. T. A matter of state: diversity in oligodendrocyte lineage cells. Neuroscientist https://doi.org/10.1177/1073858420987208 (2020).
Xiao, L. et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 19, 1210–1217 (2016).
Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science 330, 779–782 (2010).
Makinodan, M., Rosen, K. M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012). This study shows distinct regulation of myelination in specific brain regions in juvenile mice. Provides evidence for the notion that a different mechanism exists for myelination during the putative second wave of myelin changes in mice.
Scholz, J., Klein, M. C., Behrens, T. E. & Johansen-Berg, H. Training induces changes in white matter architecture. Nat. Neurosci. 12, 1370–1371 (2009). This MRI study provides evidence that motor skill learning induces white matter changes in humans.
McKenzie, I. A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014). This study provides evidence for motor skill learning evoking oligodendrogenesis in mice and that oligodendrogenesis is required for motor skill task performance.
Pfeiffer, S., Warrington, A. & Bansal, R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3, 191–197 (1993).
Bacmeister, C. M. et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 23, 819–831 (2020). This paper, along with Orthmann-Murphy et al., 2020, provides evidence for the importance of myelin patterns.
Auer, F., Vagionitis, S. & Czopka, T. Evidence for myelin sheath remodeling in the CNS revealed by in vivo imaging. Curr. Biol. 28, 549–559 (2018).
Carreiras, M. et al. An anatomical signature for literacy. Nature 461, 983–986 (2009).
Bengtsson, S. L. et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150 (2005).
Sampaio-Baptista, C. et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 (2013).
Steadman, P. E. et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164 e156 (2020).
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R. & Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23, 487–499 (2020).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Swire, M., Kotelevtsev, Y., Webb, D. J., Lyons, D. A. & ffrench-Constant, C. Endothelin signalling mediates experience-dependent myelination in the CNS. eLife https://doi.org/10.7554/eLife.49493 (2019).
Barrera, K. et al. Organization of myelin in the mouse somatosensory barrel cortex and the effects of sensory deprivation. Dev. Neurobiol. 73, 297–314 (2013).
Osanai, Y. et al. Length of myelin internodes of individual oligodendrocytes is controlled by microenvironment influenced by normal and input-deprived axonal activities in sensory deprived mouse models. Glia 66, 2514–2525 (2018).
Etxeberria, A. et al. Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J. Neurosci. 36, 6937–6948 (2016).
Chorghay, Z. et al. Activity-dependent alteration of early myelin ensheathment in a developing sensory circuit. Preprint at BioRxiv https://doi.org/10.1101/2021.04.18.440312 (2021).
Stedehouder, J., Brizee, D., Shpak, G. & Kushner, S. A. Activity-dependent myelination of parvalbumin interneurons mediated by axonal morphological plasticity. J. Neurosci. 38, 3631–3642 (2018).
Giedd, J. N. et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863 (1999).
Tripathi, R. B. et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 21, 316–323 (2017).
Foster, A. Y., Bujalka, H. & Emery, B. Axoglial interactions in myelin plasticity: evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia 67, 2038–2049 (2019).
Stadelmann, C., Timmler, S., Barrantes-Freer, A. & Simons, M. Myelin in the central nervous system: structure, function, and pathology. Physiol. Rev. 99, 1381–1431 (2019).
de Faria, O. Jr., Pama, E. A. C., Evans, K., Luzhynskaya, A. & Karadottir, R. T. Neuroglial interactions underpinning myelin plasticity. Dev. Neurobiol. 78, 93–107 (2018).
Monje, M. Myelin plasticity and nervous system function. Annu. Rev. Neurosci. 41, 61–76 (2018).
Andreae, L. C. & Burrone, J. The role of neuronal activity and transmitter release on synapse formation. Curr. Opin. Neurobiol. 27, 47–52 (2014).
Pringle, N. P., Mudhar, H. S., Collarini, E. J. & Richardson, W. D. PDGF receptors in the rat CNS: during late neurogenesis, PDGF-alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115, 535–551 (1992).
Dawson, M. R., Polito, A., Levine, J. M. & Reynolds, R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24, 476–488 (2003).
Bergles, D. E., Roberts, J. D., Somogyi, P. & Jahr, C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000). This study provides evidence that OPCs receive direct synaptic inputs from neurons.
Karadottir, R., Cavelier, P., Bergersen, L. H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005).
Kukley, M., Capetillo-Zarate, E. & Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 (2007).
Ziskin, J. L., Nishiyama, A., Rubio, M., Fukaya, M. & Bergles, D. E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 10, 321–330 (2007).
Lin, S. C. & Bergles, D. E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 7, 24–32 (2004).
Karadottir, R., Hamilton, N. B., Bakiri, Y. & Attwell, D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11, 450–456 (2008).
Benamer, N., Vidal, M., Balia, M. & Angulo, M. C. Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat. Commun. 11, 5151 (2020).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Chen, T. J. et al. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 25, 852–861 (2018).
Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Li, C. et al. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61, 732–749 (2013).
Simon, C., Gotz, M. & Dimou, L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59, 869–881 (2011).
Okuda, H. et al. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 87, 3546–3553 (2009).
Xu, T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009).
Lee, S., Chong, S. Y. C., Tuck, S. J., Corey, J. M. & Chan, J. R. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc. 8, 771–782 (2013).
Bechler, M. E., Byrne, L. & ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Rosenberg, S. S., Kelland, E. E., Tokar, E., De la Torre, A. R. & Chan, J. R. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl Acad. Sci. USA 105, 14662–14667 (2008).
Colello, R. J., Devey, L. R., Imperato, E. & Pott, U. The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J. Neurosci. 15, 7665–7672 (1995).
De Biase, L. M. et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 31, 12650–12662 (2011).
Guo, F. et al. Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J. Neurosci. 32, 639–645 (2012).
Kougioumtzidou, E. et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife https://doi.org/10.7554/eLife.28080 (2017).
Barres, B. A. & Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996).
Marisca, R. et al. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 23, 363–374 (2020).
Li, Q., Brus-Ramer, M., Martin, J. H. & McDonald, J. W. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett. 479, 128–133 (2010).
Ambronn, H. & Held, H. Beitrage zur kenntnis des nervenmarks. I. Uber entwicklung und bedeutung des nervenmarks. Arch. Anat. Physiol. 202–213 (1896).
Koudelka, S. et al. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455 (2016).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Spitzer, S. O. et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101, 459–471 (2019).
Brinkmann, B. G. et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581–595 (2008).
Taveggia, C. et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 56, 284–293 (2008).
Geraghty, A. C. et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265 (2019).
Micu, I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006).
Micu, I. et al. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 276, 41–50 (2016).
Baraban, M., Koudelka, S. & Lyons, D. A. Ca2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci. 21, 19–23 (2018). This paper provides in vivo evidence in zebrafish that during development calcium transient length and frequency leads to changes in myelin sheath length at an individual internode level.
Krasnow, A. M., Ford, M. C., Valdivia, L. E., Wilson, S. W. & Attwell, D. Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci. 21, 24–28 (2018). This paper provides in vivo evidence in zebrafish that during development neuronal activity evokes calcium transients and leads to changes in myelin sheath length at an individual internode level.
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Dutta, D. J. et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl Acad. Sci. USA 115, 11832–11837 (2018).
Noori, R. et al. Activity-dependent myelination: a glial mechanism of oscillatory self-organization in large-scale brain networks. Proc. Natl Acad. Sci. USA 117, 13227–13237 (2020).
Lubetzki, C., Zalc, B., Williams, A., Stadelmann, C. & Stankoff, B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19, 678–688 (2020).
Foerster, S., Hill, M. F. E. & Franklin, R. J. M. Diversity in the oligodendrocyte lineage: plasticity or heterogeneity? Glia 67, 1797–1805 (2019).
Gautier, H. O. et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6, 8518 (2015). This paper provides evidence that myelin regeneration is regulated by neuronal activity in vivo.
Li, Q., Houdayer, T., Liu, S. & Belegu, V. Induced neural activity promotes an oligodendroglia regenerative response in the injured spinal cord and improves motor function after spinal cord injury. J. Neurotrauma 34, 3351–3361 (2017).
Ortiz, F. C. et al. Neuronal activity in vivo enhances functional myelin repair. JCI Insight https://doi.org/10.1172/jci.insight.123434 (2019).
Prakash, R. S., Snook, E. M., Motl, R. W. & Kramer, A. F. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 1341, 41–51 (2010).
Etxeberria, A., Mangin, J. M., Aguirre, A. & Gallo, V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat. Neurosci. 13, 287–289 (2010).
Sahel, A. et al. Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Front. Cell. Neurosci. 9, 77 (2015).
Wolswijk, G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18, 601–609 (1998).
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Segel, M. et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 (2019).
Rawji, K. S. et al. Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in aging mice visualized by ex vivo live multiphoton imaging. J. Neurosci. 38, 1973–1988 (2018).
Neely, S. A. et al. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Preprint at BioRxiv https://doi.org/10.1101/2020.05.22.110551 (2020).
Snaidero, N. et al. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat. Commun. 11, 4901 (2020).
Yeung, M. S. Y. et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 (2019).
Jakel, S. et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547 (2019).
Franklin, R. J. M., Frisen, J. & Lyons, D. A. Revisiting remyelination: towards a consensus on the regeneration of CNS myelin. Semin. Cell Dev. Biol. https://doi.org/10.1016/j.semcdb.2020.09.009 (2020).
Neumann, B. et al. Problems and pitfalls of identifying remyelination in multiple sclerosis. Cell Stem Cell 26, 617–619 (2020).
Orthmann-Murphy, J. et al. Remyelination alters the pattern of myelin in the cerebral cortex. eLife https://doi.org/10.7554/eLife.56621 (2020). This paper, along with Bacmeister et al., 2020, provides evidence for the importance of myelin patterns.
Wang, F. et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486 (2020).
de Curtis, M., Garbelli, R. & Uva, L. A hypothesis for the role of axon demyelination in seizure generation. Epilepsia 62, 583–595 (2021).
Zhou, B., Zhu, Z., Ransom, B. R. & Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 26, 103–117 (2021).
Barres, B. A. et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46 (1992).
Sakry, D. et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 12, e1001993 (2014).
Thornton, M. A. & Hughes, E. G. Neuron–oligodendroglia interactions: activity-dependent regulation of cellular signaling. Neurosci. Lett. 727, 134916 (2020).
Bakiri, Y., Karadottir, R., Cossell, L. & Attwell, D. Morphological and electrical properties of oligodendrocytes in the white matter of the corpus callosum and cerebellum. J. Physiol. 589, 559–573 (2011).
Moore, S. et al. A role of oligodendrocytes in information processing. Nat. Commun. 11, 5497 (2020).
Sturrock, R. R. Myelination of the mouse corpus callosum. Neuropathol. Appl Neurobiol. 6, 415–420 (1980).
Hamilton, N. B. et al. Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65, 309–321 (2017).
Arancibia-Carcamo, I. L. et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife https://doi.org/10.7554/eLife.23329 (2017).
LaMantia, A. S. & Rakic, P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J. Neurosci. 10, 2156–2175 (1990).
Ford, M. C. et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun. 6, 8073 (2015).
Osanai, Y. et al. Rabies virus-mediated oligodendrocyte labeling reveals a single oligodendrocyte myelinates axons from distinct brain regions. Glia 65, 93–105 (2017).
Acknowledgements
We thank I. Mohorianu and O. Oniciuc for bioinformatic advice on RNA transcriptomic and GWAS analysis. We thank S. Hall for comments on the manuscript. This work was supported by the European Research Council (the European Union’s Horizon 2020 research and innovation programme grant agreement no. 771411; to R.T.K., S.T. and K.A.E.), the Paul G. Allen Frontiers Group (Allen Distinguished Investigator program no. 12076; to R.T.K. and B.V.), the Wellcome (Pathfinder Award 204488/Z/16/Z; to R.T.K. and H.P.), the UK Multiple Sclerosis Society (Centre of Excellence Award no. 132; to R.T.K. and O.F.) and the Lister Institute of Preventive Medicine (a research prize; to R.T.K.). The funders had no role in decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Faria, O., Pivonkova, H., Varga, B. et al. Periods of synchronized myelin changes shape brain function and plasticity. Nat Neurosci 24, 1508–1521 (2021). https://doi.org/10.1038/s41593-021-00917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00917-2
This article is cited by
-
Clemastine and metformin extend the window of NMDA receptor surface expression in ageing oligodendrocyte precursor cells
Scientific Reports (2024)
-
Functional myelin in cognition and neurodevelopmental disorders
Cellular and Molecular Life Sciences (2024)
-
Possible roles of deep cortical neurons and oligodendrocytes in the neural basis of human sociality
Anatomical Science International (2024)
-
Oligodendrocyte progenitor cells in Alzheimer’s disease: from physiology to pathology
Translational Neurodegeneration (2023)
-
Myelin in Alzheimer’s disease: culprit or bystander?
Acta Neuropathologica Communications (2023)