Abstract
In the nervous system, only one type of neuron–glial synapse is known to exist: that between neurons and oligodendrocyte precursor cells (OPCs), yet their composition, assembly, downstream signaling and in vivo functions remain largely unclear. Here, we address these questions using in vivo microscopy in zebrafish spinal cord and identify postsynaptic molecules PSD-95 and gephyrin in OPCs. The puncta containing these molecules in OPCs increase during early development and decrease upon OPC differentiation. These puncta are highly dynamic and frequently assemble at 'hotspots'. Gephyrin hotspots and synapse-associated Ca2+ activity in OPCs predict where a subset of myelin sheaths forms in differentiated oligodendrocytes. Further analyses reveal that spontaneous synaptic release is integral to OPC Ca2+ activity, while evoked synaptic release contributes only in early development. Finally, disruption of the synaptic genes dlg4a/dlg4b, gphnb and nlgn3b impairs OPC differentiation and myelination. Together, we propose that neuron–OPC synapses are dynamically assembled and can predetermine myelination patterns through Ca2+ signaling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All research materials are available upon request. All reagents generated in this paper will be shared by the corresponding authors upon request. All data reported in this paper will be shared by the corresponding authors upon request. Any additional information required to reanalyze the data reported in this paper is available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
All original code and custom scripts are available from the corresponding authors upon request. An example script is included in Supplementary Code 1.
References
Franklin, R. J. M. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Xiao, Y., Petrucco, L., Hoodless, L. J., Portugues, R. & Czopka, T. Oligodendrocyte precursor cells sculpt the visual system by regulating axonal remodeling. Nat. Neurosci. 25, 280–284 (2022).
Venkatesh, H. S. et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017).
Bergles, D. E. & Richardson, W. D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 8, a020453 (2016).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
de Biase, L. M. et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 31, 12650–12662 (2011).
Zonouzi, M. et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682 (2015).
Balia, M., Benamer, N. & Angulo, M. C. A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia 65, 1821–1832 (2017).
Mitew, S. et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9, 306 (2018).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Nagy, B., Hovhannisyan, A., Barzan, R., Chen, T. J. & Kukley, M. Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol. 15, e2001993 (2017).
Barron, T. & Kim, J. H. Neuronal input triggers Ca2+ influx through AMPA receptors and voltage-gated Ca2+ channels in oligodendrocytes. Glia 67, 1922–1932 (2019).
Sun, W., Matthews, E. A., Nicolas, V., Schoch, S. & Dietrich, D. Ng2 glial cells integrate synaptic input in global and dendritic calcium signals. Elife 5, e16262 (2016).
Hamilton, N., Vayro, S., Wigley, R. & Butt, A. M. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 58, 66–79 (2010).
Marisca, R. et al. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 23, 363–374 (2020).
Krasnow, A. M., Ford, M. C., Valdivia, L. E., Wilson, S. W. & Attwell, D. Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci. 21, 24–30 (2018).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Gross, G. G. et al. Recombinant probes for visualizing endogenous synaptic proteins in living neurons. Neuron 78, 971–985 (2013).
Uezu, A. et al. Identification of an elaborate complex mediating postsynaptic inhibition. Science. 353, 1123–1129 (2016).
Dempsey, W. P. et al. Regional synapse gain and loss accompany memory formation in larval zebrafish. Proc. Natl Acad. Sci. USA 119, e2107661119 (2022).
Hughes, A. N. & Appel, B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat. Commun. 10, 4125 (2019).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Spitzer, S. O. et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101, 459–471 (2019).
Yoshii, A. & Constantine-Paton, M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 10, 702–711 (2007).
Meier, J. & Grantyn, R. A gephyrin-related mechanism restraining glycine receptor anchoring at GABAergic synapses. J. Neurosci. 24, 1398–1405 (2004).
El-Husseini, A. E. -D., Schnell, E., Chetkovich, D. M., Nicoll, R. A. & Bredt, D. S. PSD-95 involvement in maturation of excitatory synapses. Science 290, 1364–1368 (2000).
Prange, O., Wong, T. P., Gerrow, K., Wang, Y. T. & El-Husseini, A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl Acad. Sci. USA 101, 13915–13920 (2004).
Saiyed, T. et al. Molecular basis of gephyrin clustering at inhibitory synapses: role of G- and E-domain interactions. J. Biol. Chem. 282, 5625–5632 (2007).
Dejanovic, B. et al. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS Biol. 12, e1001908 (2014).
Oh, W. C., Lutzu, S., Castillo, P. E. & Kwon, H. B. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 353, 1037–1040 (2016).
Fortin, D. A. et al. Live imaging of endogenous PSD-95 using ENABLED: a conditional strategy to fluorescently label endogenous proteins. J. Neurosci. 34, 16698–16712 (2014).
Fang, H., Bygrave, A. M., Roth, R. H., Johnson, R. C. & Huganir, R. L. An optimized CRISPR/Cas9 approach for precise genome editing in neurons. Elife 10, e65202 (2021).
Ifrim, M. F., Janusz-Kaminska, A. & Bassell, G. J. Development of single-molecule ubiquitination mediated fluorescence complementation to visualize protein ubiquitination dynamics in dendrites. Cell Rep. 41, 111658 (2022).
Czopka, T., ffrench-Constant, C. & Lyons, D. A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths invivo. Dev. Cell 25, 599–609 (2013).
Almeida, A. R. & Macklin, W. B. Early myelination involves the dynamic and repetitive ensheathment of axons which resolves through a low and consistent stabilization rate. Elife 12, e82111 (2023).
Kukley, M., Nishiyama, A. & Dietrich, D. The fate of synaptic input to NG2 glial cells: Neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J. Neurosci. 30, 8320–8331 (2010).
De Biase, L. M., Nishiyama, A. & Bergles, D. E. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 30, 3600–3611 (2010).
Hildebrand, D. G. C. et al. Whole-brain serial-section electron microscopy in larval zebrafish. Nature 545, 345–349 (2017).
Baraban, M., Koudelka, S. & Lyons, D. A. Ca2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci. 21, 19–25 (2018).
Lu, T. Y. et al. Norepinephrine modulates calcium dynamics in cortical oligodendrocyte precursor cells promoting proliferation during arousal in mice. Nat. Neurosci. 26, 1739–1750 (2023).
Fiore, F. et al. Norepinephrine regulates calcium signals and fate of oligodendrocyte precursor cells in the mouse cerebral cortex. Nat. Commun. 14, 8122 (2023).
Koudelka, S. et al. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455 (2016).
Shipman, S. L. et al. Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nat. Neurosci. 14, 718–726 (2011).
Osso, L. A., Rankin, K. A. & Chan, J. R. Experience-dependent myelination following stress is mediated by the neuropeptide dynorphin. Neuron 109, 3619–3632 (2021).
Almeida, R. G. et al. Myelination induces axonal hotspots of synaptic vesicle fusion that promote sheath growth. Curr. Biol. 31, 3743–3754 (2021).
Gerrow, K. et al. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron 49, 547–562 (2006).
Panzer, J. A. et al. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev. Biol. 285, 340–357 (2005).
Wierenga, C. J., Becker, N. & Bonhoeffer, T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat. Neurosci. 11, 1044–1052 (2008).
Villa, K. L. et al. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89, 756–769 (2016).
Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Proctor, D. T. et al. Axo-glial communication through neurexin–neuroligin signaling regulates myelination and oligodendrocyte differentiation. Glia 63, 2023–2039 (2015).
Stogsdill, J. A. et al. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197 (2017).
Waggener, C. T., Dupree, J. L., Elgersma, Y. & Fuss, B. CaMKIIβ regulates oligodendrocyte maturation and CNS myelination. J. Neurosci. 33, 10453–10458 (2013).
Mitra, S. K., Hanson, D. A. & Schlaepfer, D. D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/nrm1549 (2005).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015).
Almeida, R. G. & Lyons, D. A. Intersectional gene expression in zebrafish using the split KalTA4 system. Zebrafish 12, 377–386 (2015).
Meyer, M. P., Trimmer, J. S., Gilthorpe, J. D. & Smith, S. J. Characterization of zebrafish PSD-95 gene family members. J. Neurobiol. 63, 91–105 (2005).
Easley-Neal, C., Fierro, J., Buchanan, J. A. & Washbourne, P. Late recruitment of synapsin to nascent synapses is regulated by Cdk5. Cell Rep. 3, 1199–1212 (2013).
Sheets, L., Trapani, J. G., Mo, W., Obholzer, N. & Nicolson, T. Ribeye is required for presynaptic CaV1.3a channel localization and afferent innervation of sensory hair cells. Development 138, 1309–1319 (2011).
Fuhrmann, J. C. et al. Gephyrin interacts with dynein light chains 1 and 2, components of motor protein complexes. J. Neurosci. 22, 5393–5402 (2002).
Smolinsky, B., Eichler, S. A., Buchmeier, S., Meier, J. C. & Schwarz, G. Splice-specific functions of gephyrin in molybdenum cofactor biosynthesis. J. Biol. Chem. 283, 17370–17379 (2008).
Craven, S. E., El-Husseini, A. E. & Bredt, D. S. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron 22, 497–509 (1999).
Topinka, J. R. & Bredt, D. S. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron 20, 125–134 (1998).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Kwan, K. M. et al. The Tol2kit: a multisite gateway-based construction Kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 (2007).
Wyart, C. et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407–410 (2009).
Di Donato, V. et al. 2C-Cas9: a versatile tool for clonal analysis of gene function. Genome Res. 26, 681–692 (2016).
Tinevez, J. Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Srinivasan, R. et al. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717 (2015).
Acknowledgements
This work was supported by an NMSS postdoctoral fellowship FG-1907-34613 (to J.L.), a Warren Alpert Distinguished Scholar Award (to J.L.), NIH/NINDS award F31NS130898 (to T.G.M.) and NIH/NINDS grant 1R21NS120650 (to K.R.M.). We thank D. Bergles, A. Mishra, A. Nechiporuk and members of the laboratory of K.R.M. for discussions and critical reading of the manuscript. We are indebted to A. Forbes, T. Perry and G. Halsell-Vore for fish care. We thank R. Almeida, P. Brehm, M. Freeman, H. von Gersdorff, E. Gouaux, D. Lyons, L. Ma and T. Nicolson for reagents and suggestions as well as F. Coelho, S. Kaech Petrie and the OHSU microscopy core staff for feedback and assistance in imaging. We thank L. Vaskalis for graphic design.
Author information
Authors and Affiliations
Contributions
J.L. conceived the project with input from K.R.M. J.L. carried out experiments and data analyses. T.G.M. generated constructs for nlgn3a and nlgn3b disruption, GFP-tagged Nlgn3b, and truncated Nlgn3b. T.C. provided key resources before their publication. J.L. and K.R.M wrote the manuscript, and all authors edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Lucas Cheadle, Robert Hill, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
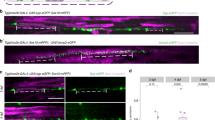
Extended Data Fig. 1 MAGUK/PSD-95, Gephyrin, and Nlgn3 are present in OPCs.
(a) RNA-seq expression of murine Dlg4 (encodes PSD-95), Gephyrin, and Nlgn3 in different CNS cell types19. Micro/Macro, Microglia and Macrophage; NFO, Newly-formed oligodendrocyte; MOL, Myelinating oligodendrocyte. n = 2; each biological replicate consists of pooled cells from a litter of 3–12 mice19. (b) Single cell RNA-seq expression of zebrafish dlg4 (a/b), gephyrin (a/b), and nlgn3 (a/b)17. VLMC, Vascular lepotomeningeal cell; MOL, Myelinating oligodendrocyte. (c) Representative IHC images of PSD-95 in Tg(olig1:Kalta4,10xUAS:myrGFP) spinal cord at 3 d.p.f. From left, spinal cord sections, 3D-reconstructed regions, and two examples of puncta in OPCs at single planes. Only the postsynaptic puncta that fall within OPC process volumes are shown in 3D-reconstructed images (green spheres) and are indicated with arrowheads in examples. In each example, from left are merged channel, OPC channel and PSD-95 channel. (d) % of puncta localized to OPC process (P) and soma (S). MAGUK, n = 18 cells from 8 fish; Gephyrin, n = 40 cells from 15 fish. All data are represented as mean ± SEM; scale bars 5 μm.
Extended Data Fig. 2 MAGUK and Gephyrin puncta numbers in OPCs increase during early development and exhibit small differences across CNS regions.
(a) Quantification of MAGUK (green) and Gephyrin (magenta) puncta number per OPC in the spinal cord at different ages by IHC. From left, n = 20, 18, 8, 17, 24, and 8 cells from at least 6 fish per condition. (b) Quantification of MAGUK (green) and Gephyrin (magenta) puncta number per OPC at 3 dpf across different CNS regions. FB&MB, forebrain and midbrain; HB, hindbrain; SC, spinal cord. From left, n = 34, 59, 43, 34, 18, and 40 cells from at least 10 fish per condition. (c) Schematic model showing the neuron-rich (NR) and axon & synapse-rich (AR) regions and two representative images showing OPCs with soma localized to NR (top) and AR (bottom) regions in Tg(olig1:Kalta4,10xUAS:myrGFP) spinal cord. (d) Quantification of MAGUK (green) and Gephyrin (magenta) puncta number per OPCs in NR or AR regions at 2-3 dpf. From left, n = 14, 5, 15, and 10 cells from at least 5 fish per condition. All data are represented as mean ± SEM; N.S., not significant (exact p values are provided in Source Data); (a-b) Kruskal-Wallis test followed by Dunn’s multiple comparison test; (d) two-sided Mann-Whitney test; scale bar 5 μm.
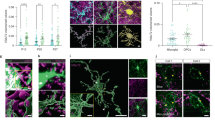
Extended Data Fig. 3 In vivo characterization of PSD-95-GFP and GFP-gephyrin.
(a) Puncta diameter of PSD-95-GFP (green) and GFP-Gephyrin (magenta) in spinal cord neurons (N) and OPCs from 2-3 dpf larvae. From left, n = 10, 11, 15, and 29 cells from at least 8 fish per condition. (b-c) Representative in vivo images of (b) PSD-95-GFP or (c) GFP-Gephyrin and synaptophysin-RFP (syn.) in spinal cord OPCs at 3 dpf. From left, single plane images and an example of puncta in OPCs at single planes with 3D reconstructed regions, the merged channels, the PSD-95-GFP or GFP-Gephyrin channels, and the syn. channel. In the examples, the alignments are indicated with arrowheads. (d) The % of PSD-95-GFP and GFP-gephyrin puncta in an OPC that align with presynaptic synaptophysin-RFP in the spinal cord in (b-c). n = 13 and 20 cells from at least 10 fish. (e-f) The correlation analysis of puncta number of (e) PSD-95-GFP and (f) GFP-Gephyrin with the corresponding protein overexpression levels at 3 dpf. The linear fit extrapolated to zero overexpression level is at puncta number of 28.6 and 22.9, respectively. (g) Puncta number of PSD-95-GFP (green) and GFP-Gephyrin (magenta) per OPC in the spinal cord at 2 dpf and 3 dpf. From left, n = 16, 18, 29, and 14 cells from at least 5 fish per condition. (h) Representative IHC images of PSD-95-GFP in spinal cord OPCs at 3 dpf with GFP (green) and MAGUK (magenta) antibodies. Left, merged channels; middle and right, single channels. Arrowheads indicate the GFP puncta that colocalize with MAGUK signals; cyan arrows indicate the diffused GFP that does not colocalize with MAGUK signals. (i) % of GFP signal that colocalizes with MAGUK signals from (h) using Imaris. n = 4 and 6 fish per condition. All data are represented as mean ± SEM; N.S., not significant (exact p values are provided in Source Data); (a,g) two-sided Mann-Whitney test; (e,f) two-sided Pearson correlation analysis with linear regression; scale bars 5 μm.
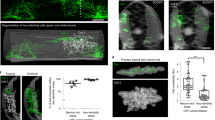
Extended Data Fig. 4 Gephyrin hotspots predict a subset of myelin sheath formation in a synaptic release-mediated manner.
(a) EM image of zebrafish spinal cord transverse section at 5 dpf40. Magenta dots indicate myelinated axons. (b) IHC images of zebrafish dorsal spinal cord transverse section at 5 dpf in Tg(mbp:GFP-caax). (c) The nearest distance between myelinated axons from (a) and (b). n = 16 and 9 sections from 1 and 6 fish; t[23] = 1.627. (d) Puncta inside hotspots (PIH) and puncta outside hotspots (POH). (e) Percentage of GFP-Gephyrin PIH and POH that predict future myelin sheaths and percentage of stable sheath volume over OPC volume with control and TeNT treatments. control, n = 8 cells from 7 fish; TeNT, n = 6 cells from 6 fish. (f) Percentage of future stable myelin sheaths predicted by hotspots in OPCs (within 1 μm). n = 6 cells from 6 fish. (g) Representative image of an OPC after ventricle injection of Dextran150,000 Antonia Red (magenta). (h-j) Quantification of (h) convex hull volume, (i) hotspot number, and (j) sheath number in control and TeNT treatment conditions. (h) n = 11 and 6 cells from 10 and 6 fish; (i) n = 18 and 6 cells from 16 and 6 fish; (j) n = 11 and 6 cells from 10 and 6 fish. (k) Representative time-lapse images of transient ensheathments. Arrowheads indicate transient sheaths; minutes. (l) Frequency distribution of hotspot-transient ensheathment distances with control and TeNT treatments. F[14, 180] = 23.87. control, n = 8 cells from 7 fish; TeNT, n = 6 cells from 6 fish. (m) Quantification of Hs- and nonHs-sheath (stable) distances with control and TeNT treatments. control, n = 8 cells from 7 fish; TeNT, n = 6 cells from 6 fish. (n) Percentage of Hs or nonHs in an OPC that predict where transient ensheathments form with control and TeNT treatments. control, n = 8 cells from 7 fish; TeNT n = 6 cells from 6 fish. All data are represented as mean ± SEM; N.S., not significant (exact p values are provided in Source Data); (c) two-sided unpaired t-test; (e) Friedman test with Dunn’s test; (f,h,i,j) two-sided Mann-Whitney test; (l) Two-way ANOVA and Fisher’s LSD test; (m,n) two-sided Wilcoxon matched-pairs signed rank test; scale bars 5 μm.
Extended Data Fig. 5 The role of synaptic release and neuronal activity in generating OPC Ca2+ activity.
(a) Basal GCaMP6s intensity and MD feret’s diameter in OPCs that neighbor few ( < 4) and many ( ≥ 4) TeNTlc-expressing axons at 5 dpf. Few, n = 14 cells from 8 fish; many, n = 26 cells from 15 fish; t[38] = 2.375 and t[38] = 2.351. (b) Normalized peak amplitude, peak duration, area of Ca2+ MD, basal intensity, and MD Feret’s diameter in OPCs before (blue) and after (magenta) injection of control or TeNT solution at 5 dpf. Paired data are indicated with grey lines. Control, n = 17 cells from 13 fish; TeNT, n = 26 cells from 18 fish. (c) Normalized peak amplitude, peak duration, and area of Ca2+ MD in OPCs before (blue) and after (magenta) injection of control or TTX solution at 5 dpf. Paired data are indicated with grey lines. From left, n = 10 and 11 cells from 6 and 9 fish. (d) Representative images of a primary motor neuron visualized by injecting mnx1:gal4 into Tg(10xUAS:myrGCaMP6s). (e) Normalized frequency of Ca2+ activity in motor neurons before (blue) and after (magenta) injection of control or TTX solution at 5 dpf. n = 7 and 6 cells from 5 and 5 fish. (f) Normalized peak amplitude, peak duration, and area of Ca2+ MD in OPCs before (blue) and after (magenta) injection of control or TTX solution at 3 dpf. Paired data are indicated with grey lines. From left, n = 10 and 24 cells from 7 and 13 fish. All data are represented as mean ± SEM; N.S., not significant (exact p values are provided in Source Data); (a) two-sided unpaired t-test or (b,c,e,f) two-sided Wilcoxon matched-pairs signed rank test; scale bars 5 μm.
Extended Data Fig. 6 Cell-specific knockdown system is efficient to disrupt genes in oligodendrocytes in zebrafish.
(a) Schematic of the plasmid (10xUAS:myrmScarlet-p2A-Cas9, U6:sgRNA1;U6:sgRNA2) used to induce cell-specific Cas9 expression with membrane labeling and 2 separate U6-driven sgRNAs. (b) Representative gel images of digested PCR product of genomic regions targeted by the sgRNAs. Each lane represents a single embryo at 1 dpf. The left 4 lanes are uninjected (-) and the right 4 lanes are injected (+) with sgRNAs and Cas9 protein. (c) Schematic of larva with sparsely labeled oligodendrocyte lineage cells resulting from Tg(sox10:Kalta4) crossed with transgenic fish carrying the plasmid from (a). (d) Representative images of single oligodendrocytes in the spinal cord of Tg(10xUAS:myrmScarlet-p2A-Cas9, U6:ctrl-sgRNA1;U6:ctrl-sgRNA2) and Tg(10xUAS:myrmScarlet-p2A-Cas9, U6:myrfex3-sgRNA;U6:myrfex4-sgRNA) at 6 dpf. Knockdown of Myrf serves as a control for our targeting approach. (e) Average myelin sheath length in (d). n = 24 and 22 cells from 15 fish; t[44] = 2.247. All data are represented as mean ± SEM; (e) two-sided unpaired t-test; scale bars 5 μm.
Extended Data Fig. 7 Nlgn3 is localized to synapses and knock-down reduces postsynaptic puncta in OPCs.
(a) Representative single-plane IHC images of Nlgn3 (Af101058) in OPCs with presynaptic marker SV2 in Tg(olig1:myrmScarlet) spinal cord at 5 dpf. From left, spinal cord sections and two alignment examples with SV2. In each example, from left are 3D reconstructed regions, merged channels, OPC channel, Nlgn3 channel, and SV2 channel. Postsynaptic puncta (blue spheres or arrowheads) and aligned SV2 (green spheres) are indicated. (b) The number of Nlgn3 puncta in an OPC that align with presynaptic SV2 in the spinal cord at 5 dpf from control and F0 larvae with nlgn3a and nlgn3b knocked down by sgRNA targeting nlgn3aex4 and nlgn3bex1 and Cas9 protein. From left, n = 6 and 7 cells from at least 5 fish each condition. (c) Representative in vivo images of Nlgn3b-GFP and synaptophysin-RFP (syn.) in the spinal cord at 5 dpf. From left, single plane image and two examples of puncta in OPCs with 3D reconstructed regions, merged channels, Nlgn3b-GFP channel, and the syn. channel. The aligned Nlgn3b puncta are indicated with arrowheads. (d) The Number of Nlgn3b-GFP puncta in OPCs that align with synaptophysin-RFP puncta. n = 6 cells from 6 fish. (e) Representative IHC images of MAGUK and Gephyrin in the spinal cord at 3 dpf with ctrl- and nlgn3b-targeted for cell-specific knockdown. From left, spinal cord sections and 3D-reconstructed images of enlarged regions. In 3D-reconstructed images, only the postsynaptic puncta that fall within OPC process volume are shown (green sphere). (f) Density of MAGUK and Gephyrin puncta in OPCs from (e). From left, n = 12, 12, 16, and 16 cells from at least 8 fish. (g) Representative in vivo images of an OPC expressing the cell-specific knockdown construct in Tg(olig1:Kalta4, olig1:myrGCaMP6s) at 4 dpf. (h-i) The frequency of (h) Ca2+ activity and (i) MD number in ctrl- and nlgn3b-targeted OPCs at 3-4 dpf. ctrl, n = 13 cells from 13 fish; nlgn3b, n = 17 cells from 16 fish; t[28] = 2.260 and t[28] = 2.511. All data are represented as mean ± SEM; (b,f) two-sided Mann-Whitney test; (h,i) two-sided unpaired t-test; scale bars 5 μm.
Extended Data Fig. 8 Disruption of nlgn3b impairs OPC development and myelination.
(a-b) Number of myelin sheaths per oligodendrocyte in ctrl-, dlg4ab-, gphnb-, nlgn3a-, and nlgn3b-targeted oligodendrocytes at (a) 3 dpf and (b) 5dpf. a, n = 121, 50, 34, 30, and 170 cells from 78, 30, 27, 26, and 97 fish; b, n = 118, 52, 63, 80, and 152 cells from 80, 36, 40, 61, and 93 fish. (c) Schematic showing the domains of Nlgn3b and the region mutated in the dominant-negative construct employed in our studies. (d) 4 single planes of a trapezoid-shaped process in an oligodendrocyte in nlgn3b-targeted larvae at 5 dpf. Myelin sheaths are indicated by black arrowheads and abnormal processes by magenta arrowheads. (e) Percentage of oligodendrocytes that possess both myelin sheath(s) and abnormal processes in ctrl-, nlgn3a-, and nlgn3b-targeted fish at 5 dpf. n = 119, 84, and 170 cells from 80, 61, and 93 fish, F[6] = 13.52. All data are represented as mean ± SEM; N.S., not significant (exact p values are provided in Source Data); (a,b,e) Kruskal-Wallis test followed by uncorrected Dunn’s multiple comparisons test; scale bars 5 μm.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Table 1
Supplementary Video 1
Representative video of 10xUAS:GFP–gephyrin in an OPC and a neuron next to each other in the spinal cord of a Tg(sox10:Kalta4) larva at 3 d.p.f. The video was generated through maximum projection of stacks.
Supplementary Video 2
Representative video of 10xUAS:GFP–gephyrin in an OPC that eventually differentiates in the spinal cord of a Tg(sox10:Kalta4, 10xUAS:myrmScarlet) larva at 3 d.p.f. The video was generated through maximum projection of stacks.
Supplementary Video 3
Representative video of Ca2+ activity in an OPC in a Tg(olig1:Kalta4,10xUAS:myrGCaMP6s) larva at 5 d.p.f.
Supplementary Video 4
Video of Ca2+ activity in an OPC with multiple events including a somal event in a Tg(olig1:Kalta4,10xUAS:myrGCaMP6s) larva at 5 d.p.f.
Supplementary Video 5
Representative 10-min video of Ca2+ activity in an OPC before (left) and after (right) injection of TeNT in a Tg(olig1:Kalta4,10xUAS:myrGCaMP6s) larva at 5 d.p.f.
Supplementary Data 1
Statistical source data for Supplementary Fig. 1.
Supplementary Code 1
Calcium analysis example MATLAB script.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Miramontes, T.G., Czopka, T. et al. Synaptic input and Ca2+ activity in zebrafish oligodendrocyte precursor cells contribute to myelin sheath formation. Nat Neurosci 27, 219–231 (2024). https://doi.org/10.1038/s41593-023-01553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01553-8