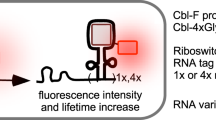
Abstract
Fluorescent RNAs, aptamers that bind and activate small fluorogenic dyes, have provided a particularly attractive approach to visualizing RNAs in live cells. However, the simultaneous imaging of multiple RNAs remains challenging due to a lack of bright and stable fluorescent RNAs with bio-orthogonality and suitable spectral properties. Here, we develop the Clivias, a series of small, monomeric and stable orange-to-red fluorescent RNAs with large Stokes shifts of up to 108 nm, enabling the simple and robust imaging of RNA with minimal perturbation of the target RNA’s localization and functionality. In combination with Pepper fluorescent RNAs, the Clivias enable the single-excitation two-emission dual-color imaging of cellular RNAs and genomic loci. Clivias can also be used to detect RNA–protein interactions by bioluminescent imaging both in live cells and in vivo. We believe that these large Stokes shift fluorescent RNAs will be useful tools for the tracking and quantification of multiple RNAs in diverse biological processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the paper and its Supplementary Information files. Source data are provided with this paper.
References
Enterina, J. R., Wu, L. & Campbell, R. E. Emerging fluorescent protein technologies. Curr. Opin. Chem. Biol. 27, 10–17 (2015).
Shcherbakova, D. M., Hink, M. A., Joosen, L., Gadella, T. W. & Verkhusha, V. V. An orange fluorescent protein with a large Stokes shift for single-excitation multicolor FCCS and FRET imaging. J. Am. Chem. Soc. 134, 7913–7923 (2012).
Kogure, T. et al. A fluorescent variant of a protein from the stony coral Montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat. Biotechnol. 24, 577–581 (2006).
Yang, J. et al. mBeRFP, an improved large Stokes shift red fluorescent protein. PLoS One 8, e64849 (2013).
Piatkevich, K. D. et al. Monomeric red fluorescent proteins with a large Stokes shift. Proc. Natl Acad. Sci. USA 107, 5369–5374 (2010).
Chu, J. et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 34, 760–767 (2016).
Paige, J. S., Wu, K. Y. & Jaffrey, S. R. RNA mimics of green fluorescent protein. Science 333, 642–646 (2011).
Strack, R. L., Disney, M. D. & Jaffrey, S. R. A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat-containing RNA. Nat. Methods 10, 1219–1224 (2013).
Filonov, G. S., Moon, J. D., Svensen, N. & Jaffrey, S. R. Broccoli: rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J. Am. Chem. Soc. 136, 16299–16308 (2014).
Dolgosheina, E. V. et al. RNA mango aptamer-fluorophore: a bright, high-affinity complex for RNA labeling and tracking. ACS Chem. Biol. 9, 2412–2420 (2014).
Sunbul, M. & Jaschke, A. SRB-2: a promiscuous rainbow aptamer for live-cell RNA imaging. Nucleic Acids Res. 46, e110 (2018).
Arora, A., Sunbul, M. & Jaschke, A. Dual-colour imaging of RNAs using quencher- and fluorophore-binding aptamers. Nucleic Acids Res. 43, e144 (2015).
Sunbul, M. & Jaschke, A. Contact-mediated quenching for RNA imaging in bacteria with a fluorophore-binding aptamer. Angew. Chem. Int. Ed. Engl. 52, 13401–13404 (2013).
Autour, A. et al. Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat. Commun. 9, 656 (2018).
Li, X., Kim, H., Litke, J. L., Wu, J. & Jaffrey, S. R. Fluorophore-promoted RNA folding and photostability enables imaging of single Broccoli-tagged mRNAs in live mammalian cells. Angew. Chem. Int. Ed. Engl. 59, 4511–4518 (2020).
Li, X. et al. Imaging intracellular S-adenosyl methionine dynamics in live mammalian cells with a genetically encoded red fluorescent RNA-based sensor. J. Am. Chem. Soc. 142, 14117–14124 (2020).
Tan, X. et al. Fluoromodules consisting of a promiscuous RNA aptamer and red or blue fluorogenic cyanine dyes: selection, characterization, and bioimaging. J. Am. Chem. Soc. 139, 9001–9009 (2017).
Braselmann, E. et al. A multicolor riboswitch-based platform for imaging of RNA in live mammalian cells. Nat. Chem. Biol. 14, 964–971 (2018).
Bouhedda, F. et al. A dimerization-based fluorogenic dye-aptamer module for RNA imaging in live cells. Nat. Chem. Biol. 16, 69–76 (2020).
Song, W. et al. Imaging RNA polymerase III transcription using a photostable RNA–fluorophore complex. Nat. Chem. Biol. 13, 1187–1194 (2017).
Cawte, A. D., Unrau, P. J. & Rueda, D. S. Live cell imaging of single RNA molecules with fluorogenic Mango II arrays. Nat. Commun. 11, 1283 (2020).
Sunbul, M. et al. Super-resolution RNA imaging using a rhodamine-binding aptamer with fast exchange kinetics. Nat. Biotechnol. 39, 686–690 (2021).
Dey, S. K. et al. Publisher correction: Repurposing an adenine riboswitch into a fluorogenic imaging and sensing tag. Nat. Chem. Biol. 18, 236 (2022).
Wirth, R., Gao, P., Nienhaus, G. U., Sunbul, M. & Jaschke, A. SiRA: a silicon rhodamine-binding aptamer for live-cell super-resolution RNA imaging. J. Am. Chem. Soc. 141, 7562–7571 (2019).
Zhang, J., Wang, L., Jaschke, A. & Sunbul, M. A color-shifting near-infrared fluorescent aptamer-fluorophore module for live-cell RNA imaging. Angew. Chem. Int. Ed. Engl. 60, 21441–21448 (2021).
Li, X., Wu, J. & Jaffrey, S. R. Engineering fluorophore recycling in a fluorogenic RNA aptamer. Angew. Chem. Int. Ed. Engl. 60, 24153–24161 (2021).
Chen, X. et al. Visualizing RNA dynamics in live cells with bright and stable fluorescent RNAs. Nat. Biotechnol. 37, 1287–1293 (2019).
Steinmetzger, C., Palanisamy, N., Gore, K. R. & Hobartner, C. A multicolor large Stokes shift fluorogen-activating RNA aptamer with cationic chromophores. Chemistry 25, 1931–1935 (2019).
Huang, K. et al. Structure-based investigation of fluorogenic Pepper aptamer. Nat. Chem. Biol. 17, 1289–1295 (2021).
Yuan, L., Lin, W. & Chen, H. Analogs of Changsha near-infrared dyes with large Stokes shifts for bioimaging. Biomaterials 34, 9566–9571 (2013).
Grubbs, R. D. Intracellular magnesium and magnesium buffering. Biometals 15, 251–259 (2002).
Prasanth, K. V. et al. Nuclear organization and dynamics of 7SK RNA in regulating gene expression. Mol. Biol. Cell 21, 4184–4196 (2010).
Marnef, A., Richard, P., Pinzon, N. & Kiss, T. Targeting vertebrate intron-encoded box C/D 2'-O-methylation guide RNAs into the Cajal body. Nucleic Acids Res. 42, 6616–6629 (2014).
Novotny, I. et al. SART3-dependent accumulation of incomplete spliceosomal snRNPs in Cajal bodies. Cell Rep. 10, 429–440 (2015).
Tsalikis, J. et al. Intracellular bacterial pathogens trigger the formation of U small nuclear RNA bodies (U Bodies) through metabolic stress induction. J. Biol. Chem. 290, 20904–20918 (2015).
Jain, S. et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Shao, S. et al. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res. 44, e86 (2016).
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Xiong, F. & Frasch, W. D. OmegaqPCR measures telomere length from single-cells in base pair units. Nucleic Acids Res. 49, e120 (2021).
Hall, M. P. et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 7, 1848–1857 (2012).
Johansson, H. E. et al. A thermodynamic analysis of the sequence-specific binding of RNA by bacteriophage MS2 coat protein. Proc. Natl Acad. Sci. USA 95, 9244–9249 (1998).
Grahn, E. et al. Structural basis of pyrimidine specificity in the MS2 RNA hairpin-coat-protein complex. RNA 7, 1616–1627 (2001).
Franklin, N. C. ‘N’ transcription antitermination proteins of bacteriophages lambda, phi 21 and P22. J. Mol. Biol. 181, 85–91 (1985).
Loughlin, F. E. et al. The zinc fingers of the SR-like protein ZRANB2 are single-stranded RNA-binding domains that recognize 5′ splice site-like sequences. Proc. Natl Acad. Sci. USA 106, 5581–5586 (2009).
Kondo, Y., Oubridge, C., van Roon, A. M. & Nagai, K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5′ splice site recognition. eLife 4, e04986 (2015).
Spritz, R. A., Strunk, K., Surowy, C. S. & Mohrenweiser, H. W. Human U1-70K ribonucleoprotein antigen gene: organization, nucleotide sequence, and mapping to locus 19q13.3. Genomics 8, 371–379 (1990).
Wu, R. et al. Ratiometric fluorogenic RNA-based sensors for imaging live-cell dynamics of small molecules. ACS Appl. Bio Mater. 3, 2633–2642 (2020).
Konig, K. Multiphoton microscopy in life sciences. J. Microsc. 200, 83–104 (2000).
Protter, D. S. W. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
Liu, R. et al. Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins. Nat. Biotechnol. 40, 779–786 (2022).
Litke, J. L. & Jaffrey, S. R. Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts. Nat. Biotechnol. 37, 667–675 (2019).
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2022YFC3400100 to Y.Y. and X.C., 2019YFA0110500 to L.Z., 2019YFA0904800 to Y.Y. and L.Z. and 2021YFC2300300 to A.R.), NSFC (91857202, 32121005, 32150028 and 21937004 to Y.Y.; 21907029 and 21877037 to L.Z.; 32022039, 31870810, 91940302 and 91640104 to A.R.; 32250009 to X.C.; 32001051 to N.S.), STI2030-Major Projects (2021ZD0202200 and 2021ZD0202203 to X.C.), the Shanghai Municipal Education Commission (2021 Sci and Tech 03-28), ALS Project from Shanghai Jiao Tong University School of Medicine Affiliated Sixth People’s Hospital (to Y.Y.), the Shanghai Rising-Star Program to X.C., the State Key Laboratory of Bioreactor Engineering (to Y.Y.), China Postdoctoral Science Foundation (2022M711147 to X. Xie.), and the Fundamental Research Funds for the Central Universities (to Y.Y. and X.C.).
Author information
Authors and Affiliations
Contributions
Concepts were conceived by Y.Y., L.Z., X.C. and A.R.; Y.Y., L.Z., X.C., L.J., D.Z., X. Xie and N.S. designed the experiments and analyzed the data; L.J. and D.Z. synthesized the dyes, X.C. performed the SELEX experiment, X.C., X. Xie, X. Xu, F.Z., M.F., J.Y., R.Z., H.L. and B.Z. characterized the aptamer in vitro and constructed plasmids, and N.S. and X. Xie performed the live cell imaging experiments. K.H., B.B., L.Y., X.H., Q.Z., Z.C., R.L., Q.L. and Y.Z. gave technical support and conceptual advice. Y.Y., L.Z., A.R. and X.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Y.Y., X.C., X. Xie and N.S. are named inventors of patent application number 202010206404.1. All other authors have no competing interests.
Peer review
Peer review information
Nature Methods thanks Gal Haimovich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Screening, optimization and characterization of Clivia.
(a) Excitation and emission spectra of NBSI in glycerol. NBSI showed minimal fluorescence in water, but emitted bright fluorescence in glycerol. Δλ represents the Stokes shift. (b) The Mfold-predicted secondary structure of R8 aptamer. (c) Fluorescence activation of NBSI by R8 aptamer. (d) The excitation and emission spectra of R8-NBSI complex and NBSI alone. (e) FACS analysis of R8-NBSI complexes in BL21 Star™ (DE3) cells. E. coli. BL21 Star™ (DE3) cells expressing tRNA-R8 were induced by adding IPTG at 37 °C for 4 h. The cells were collected and incubated with 0.5 µM NBSI in HEPES buffer (containing 5 mM Mg2+). Fluorescence was analyzed using a flow cytometry with a 561 nm excitation. The gate (blue) was placed based on the BL21 Star™ (DE3) cells transformed with empty vector and incubated with 0.5 µM NBSI to determine population fraction that expressed tRNA-R8. (f) The Mfold-predicted secondary structures of R8 and its truncation mutants. (g) Quantification of NBSI fluorescence induced by R8 and its truncation mutants. The markedly reduced fluorescence of the T5 aptamer suggests that T4 is the minimal aptamer sequence required for activation of NBSI fluorescence. (h) Stem-loop indicated in R8-T4 structure was replaced by other stem-loops with difference sequences. (i) Quantification of fluorescence of R8-T4 with different stem-loops. (j) Mutation sites (purple nucleotides) in T4-2. (k) Quantification of fluorescence of T4-2 mutants. (l) Thermostability of different T4-2 mutants. (m) Potassium independence for Clivia-induced fluorescence. (n) Schematic representation of different tandem arrays of Clivia. (o) Live cell imaging of different tandem arrays of Clivia in HEK293T cells. The cells were incubated with 0.2 µM NBSI for 10 min before imaging. Scale bar, 10 μm. (p) Quantification analysis of the fluorescence for different Clivia arrays in live cells. Data represent the mean ± s.d. (n = 200 cells). Data in (c), (g), (i), (k), (l), (m) represent the mean ± s.d. from three biologically independent replicates.
Extended Data Fig. 2 Strategy to tune the spectra of fluorophores.
NBSI has a typical dye molecular structure, including electron donor, electron acceptor and π conjugate structure. Thus, it is possible to fine tune the photophysical properties of NBSI by adjusting the length of π conjugate structure, the electron withdrawing capability of the electron acceptor group or the electron donating capability of the electron donor group. To keep the large Stokes shifts of the fluorophores, two strategies were conducted to fine tune the spectra characteristics of the fluorophores: Strategy 1: substitute the electron acceptor group by introducing different substituents on the benzene to adjust the electron withdrawing ability of NBSI. Strategy 2: substitute the electron donor group with phenol hydroxyl group and meanwhile introduce two fluorine atoms at its adjacent positions. Then, substitute the electron acceptor group described in strategy 1.
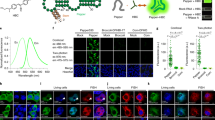
Extended Data Fig. 3 A palette of Clivias FRs with large Stokes shifts.
(a) Structures of NBSI-like fluorophores. (b) Excitation (dashed) and emission (solid) spectra of the Clivias and their Stokes shifts were indicated. (c) Imaging of HEK293T cells expressing 4Clivia and incubated with 0.5 μM various NBSI-like fluorophores. Cells transfected with the empty vector were used as controls. The nucleus was stained with 1 μg/ml Hoechst 33342 (blue). Scale bars, 10 μm. (d) Quantification analysis of the fluorescence for different RNA–fluorophore complexes in live cells. The data were normalized to the average fluorescence of each RNA–fluorophore complex, respectively. Data represent the mean ± s.d. (n = 200 cells).
Extended Data Fig. 4 Photostability of different FRs and FP in single-photon excitation.
(a) HEK293T cells expressing tRNA-Clivia or tRNA-Chili were labeled with 0.5 μM NBSI and its analogs or 1 μM DMHBI-lmi. Continuous fluorescence imaging was performed using a spinning disk confocal laser scanning microscope with a 488 nm single-photon excitation. Cells expressing CyOFP1-H2B were used as the controls. Scale bars, 10 μm. (b) Quantification of the fluorescence in (a). Data were normalized to the initial image intensity (at time 0), N=10 cells.
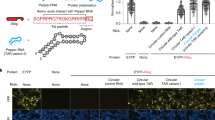
Extended Data Fig. 5 Dual-color imaging of two RNAs using Clivia and Pepper.
(a) The bio-orthogonality of Clivias with Peppers. Activation of HBC or NBSI ligands by Pepper or Clivia using 1 μM ligands and 5 μM aptamer. Data represent the mean ± s.d. from three biologically independent replicates. (b) The excitation and emission spectra of Clivia595 and Pepper497. (c) Schematic representation of the protein–RNA tethered reporting system based on the interaction of MCP and the MS2 binding hairpin RNA, as well as λN and the boxB-binding hairpin RNA. (d) Imaging of HEK293T cells expressing Clivia-MS2, tdMCP-SNAP-H2B, Pepper-boxB and N-TOMM20-BFP that targeted the nucleus and outer mitochondrial membrane, respectively, using a single excitation in single-photon and two-photon microcopy. The cells were labeled with 0.5 μM NBSI595, 1 μM HBC497 and 0.2 μM SNAP-Cell 647-SiR. Scale bar, 10 μm. (e) Single-excitation two-emission dual-color imaging of two tethered RNAs using different Clivia and Pepper combinations. HEK293T cells were transfected with plasmids expressing Clivia-MS2, MCP-SNAP-H2B, Pepper-boxB and λN-TOMM20-BFP that targeted to nucleus and outer mitochondrial membrane, respectively. Thirty-six hrs after transfection, the cells were incubated with HBC497 and NBSI570, HBC497 and NBSI571, HBC497 and NBSI574, HBC497 and NBSI578 or HBC530 and NBSI618. Scale bars, 10 μm. (f) Single-excitation two-emission dual-color imaging of U6 snRNA and ACTB mRNA using Clivia and Pepper in single-photon or two-photon microcopy, respectively. Scale bars, 10 μm.
Extended Data Fig. 6 U-body puncta formation in HEK293T cells after thapsigargin induction.
(a) Thapsigargin-induced U-body formation. Scale bar, 10 μm. (b) HEK293T cells expressing U1-Clivia were treated with thapsigargin and consecutive imaging was taken every 10 min. Scale bar, 10 μm. (c) Zoom-in of the white square indicated region from (b) to show the fusion of small U-body puncta to form big U-body puncta. The # 1 and # 2 U-body puncta were fused to form the # 4 U-body punctum, and then the # 3 and # 4 U-body puncta were fused to form the # 5 U-body punctum. Scale bar, 5 μm.
Extended Data Fig. 7 Determination of the telomere length based on signal intensity of Pepper-tagged sgRNA.
(a) Imaging of genomic foci with varied copy number by sgRNA-Pepper. Pepper-tagged sgRNAs targeting to genomic loci with varied copy number: C13-1 (177 copies) located in chromosome 13, PR1 (160 copies), CACNG7 (99 copies), T2 (77 copies) and IDR4 (51 copies) located in chromosome 19 were constructed and used for genomic labeling. Zoom-in of the white square indicated regions was shown. Scale bars, 5 μm. (b) The standard curve by plotting the fluorescent intensities on the x-axis and the copy number of genomic locus on the y-axis. The data were fit to a linear equation and the R2 value was calculated. Data represent the mean ± s.d. (n = 10 cells). (c) Quantification analysis of the copy number of telomeres in a HEK293T cells. Scale bars, 5 μm. (d) Calculation of the copy number of telomeres from their fluorescent signals based on the equation in (c).
Extended Data Fig. 8 The effect of different configurations of MS2-MCP complex on the BRET efficiencies.
(a) Structure information showing the interaction between MCP coat protein and MS2 hairpin RNA (PDB: 2C50). Amino acids in MCP protein dimer that participate in the binding of MS2 RNA are shown. (b) Schematic representation of the RNA reporter and BRET fusion proteins. (c) Emission spectra measurement of HEK293T cells expressing Clivia-MS2 and NLuc fusions upon incubation with 1 μM NBSI574 and 10 μM furimazine. Cells expressing Clivia-MS2 and NLuc without fusion with MCP were used as the control. Data were normalized to the maximal emission. (d) Schematic representation of the binding models of different BRET fusion proteins to Clivia-MS2 reporter RNA. (e) BRET efficiency for different BRET fusion proteins. HEK293T cells expressing Clivia-MS2 and different BRET fusion proteins were incubated with 1 μM NBSI574 and 10 μM furimazine, and the emission spectra were measured. The ratios of light intensity at the emission maximum of Clivia to that of NLuc were calculated. Cells expressing Clivia-MS2 and NLuc without fusion with MCP were used as the controls. Data represent the mean ± s.d. from three biologically independent samples. (f) Schematic representation of the different BRET fusion proteins. (g) Schematic representation of the binding models of different BRET fusion proteins to Clivia-MS2 reporter RNA. In the first model, NLuc-dMCP can bind with Clivia-MS2 reporter RNA in two possible configurations and thus have different BRET efficiencies. In the second model, NLuc-dMCP (Y85G/N55G/K57G/E63G) contains the configuration-specific mutations in dMCP motif and may bind with MS2 reporter RNA in the configuration favored more efficient BRET. In the third model, NLuc-cpdMCP (N55G/K57G/E63G/Y85G) also contains the configuration-specific mutations in dMCP motif and may bind with MS2 reporter RNA in the unfavored configuration with less efficient BRET. (h) BRET efficiency for different BRET fusion proteins. Data represent the mean ± s.d. from three biologically independent replicates. (i) Schematic representation of NLuc-ddMCP fusions containing different lengths of amino acid linkage between NLuc and ddMCP. (j) Schematic representation of the chimeric Clivia-MS2 reporter RNA containing different lengths of nucleotide stem between Clivia and MS2.
Extended Data Fig. 9 Detection of U1-70K and U1 snRNA interaction by the Clivia-based BRET system.
(a) Schematic illustration of different configurations of U1 snRNA with Clivia (red) insertion. (b) Schematic representation of different chimeric Clivia-U1 reporters and the fusion of NLuc and the RNA binding domain (amino acids 1-194) of U1-70K. (c) Validation of different chimeric Clivia-U1 reporter RNA for labeling of U-bodies induced by thapsigargin. Scale bars, 10 μm. (d) The BRET efficiency between U1-70K(1-194)-NLuc and different chimeric Clivia-U1 RNA reporters. Cells expressing U1-70K(1-194)-NLuc and Clivia were used as the controls. Statistical comparison was performed by a two-tailed t-test. **P < 0.01 and ***P < 0.001 versus control cells. N.S., no significant difference. Data represent the mean ± s.d. from four biologically independent replicates. (e) Imaging of HEK293T cells expressing U1-70K(1-194)-NLuc and different chimeric Clivia-U1 RNA reporters upon incubation with 1 μM NBSI574 and 10 μM furimazine. Cells expressing U1-70K(1-194)-NLuc and Clivia were used as the controls. Scale bars, 40 μm.
Extended Data Fig. 10 Detection of U1-70K and U1 snRNA interaction in U-bodies and cells during interphase and mitotic phase.
(a) Schematic illustration of Clivia-based BRET system for detection of the interaction between U1-70K and U1 snRNA. (b) Schematic representation of the fusions of NLuc and different U1-70K truncations. (c) The BRET efficiencies between Clivia-U1-SL3 and different fusion proteins. Cells expressing Clivia-U1-SL3 and NLuc were used as the controls. Data represent the mean ± s.d. from three biologically independent replicates. (d) Imaging of HEK293T cells expressing Clivia-U1-SL3 and different fusion proteins upon incubation with 1 μM NBSI574 and 10 μM furimazine. Cells expressing Clivia-U1-SL3 and NLuc were used as the controls. Scale bars, 40 μm. (e) Imaging of the BRET signals in the nucleoplasm and U-bodies. Scale bars, 10 μm. (f) Quantification of the BRET ratios in the nucleoplasm and U-bodies. Data represent the mean ± s.d. (N = 30 cells). (g) Imaging of the BRET signals in the cells during interphase and mitotic phase. HEK293T cell were co-transfected with plasmids expressing Clivia-U1-SL3, U1-70K(1-194)-NLuc and LaminA-SNAP. The cells were incubated with 1 μM NBSI574 and 0.2 μM SNAP-Cell 647-SiR 24 h after transfection. Then BRET imaging was performed after 10 μM furimazine was added. LaminA-SNAP was used to visualize the status of nuclear envelope to indicate the stage of the cell cycle. During mitosis, the nuclear envelope breaks apart in prometaphase and reforms in late mitosis. Blue arrows indicated the cells during interphase and white arrow indicated the cell during mitotic phase. Scale bars, 20 μm. (h) Quantification of the BRET ratios in the cells during interphase and mitotic phase. Data represent the mean ± s.d. (N = 20 cells). Statistical comparisons in (c), (f) and (h) were performed by a two-tailed t-test. ***P < 0.001 versus control cells. N.S., no significant difference.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Supplementary Tables 1–4, Supplementary Notes 1–4 and Supplementary References.
Supplementary Video 1
Real-time monitoring of U-body formation after thapsigargin induction.
Supplementary Video 2
Fusion of small U-bodies to form big ones.
Supplementary Video 3
Real-time monitoring of the movement of the genomic loci.
Supplementary Data 1
Source data for Supplementary Figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, L., Xie, X., Su, N. et al. Large Stokes shift fluorescent RNAs for dual-emission fluorescence and bioluminescence imaging in live cells. Nat Methods 20, 1563–1572 (2023). https://doi.org/10.1038/s41592-023-01997-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01997-7
This article is cited by
-
A dual-selective thermal emitter with enhanced subambient radiative cooling performance
Nature Communications (2024)