Abstract
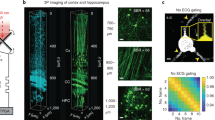
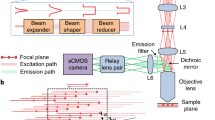
Optical imaging through the intact mouse skull is challenging because of skull-induced aberrations and scattering. We found that three-photon excitation provided improved optical sectioning compared with that obtained with two-photon excitation, even when we used the same excitation wavelength and imaging system. Here we demonstrate three-photon imaging of vasculature through the adult mouse skull at >500-μm depth, as well as GCaMP6s calcium imaging over weeks in cortical layers 2/3 and 4 in awake mice, with 8.5 frames per second and a field of view spanning hundreds of micrometers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
McGonigle, P. Biochem. Pharmacol. 87, 140–149 (2014).
Holtmaat, A. et al. Nat. Protoc. 4, 1128–1144 (2009).
Yang, G., Pan, F., Parkhurst, C. N., Grutzendler, J. & Gan, W.-B. Nat. Protoc. 5, 201–208 (2010).
Drew, P. J. et al. Nat. Methods 7, 981–984 (2010).
Dorand, R. D., Barkauskas, D. S., Evans, T. A., Petrosiute, A. & Huang, A. Y. Intravital 3, e29728 (2014).
Rangroo Thrane, V. et al. Sci. Rep. 3, 2582 (2013).
Jonckers, E., Shah, D., Hamaide, J., Verhoye, M. & Van der Linden, A. Front. Pharmacol. 6, 231 (2015).
Wang, X., Pang, Y., Ku, G., Stoica, G. & Wang, L. V. Opt. Lett. 28, 1739–1741 (2003).
Wang, R. K. et al. Opt. Express 15, 4083–4097 (2007).
Hong, G. et al. Nat. Photonics 8, 723–730 (2014).
Silasi, G., Xiao, D., Vanni, M. P., Chen, A. C. N. & Murphy, T. H. J. Neurosci. Methods 267, 141–149 (2016).
Steinzeig, A., Molotkov, D. & Castrén, E. PLoS One 12, e0181788 (2017).
Theer, P. & Denk, W. J. Opt. Soc. Am. A. Opt. Image. Sci. Vis. 23, 3139–3149 (2006).
Park, J.-H., Sun, W. & Cui, M. Proc. Natl Acad. Sci. USA 112, 9236–9241 (2015).
Zhao, Y.-J. et al. Light Sci. Appl. 7, 17153 (2018).
Zhang, C. et al. Theranostics 8, 2696–2708 (2018).
Horton, N. G. et al. Nat. Photonics 7, 205–209 (2013).
Ouzounov, D. G. et al. Nat. Methods 14, 388–390 (2017).
Wang, Y. et al. ACS Nano 11, 10452–10461 (2017).
Ascenzi, A. & Fabry, C. J. Biophys. Biochem. Cytol. 6, 139–142 (1959).
Tuchin, V. V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis 2nd ed. 143–256 (SPIE, Bellingham, WA, 2000).
Horton, N. G. & Xu, C. Biomed. Opt. Express 6, 1392–1397 (2015).
Podgorski, K. & Ranganathan, G. bioRxiv Preprint at https://www.biorxiv.org/content/early/2016/06/06/057364 (2016).
Yuryev, M. et al. Front. Cell. Neurosci. 9, 500 (2016).
Acknowledgements
We thank members of the Xu group and Schnitzer lab for discussion. We thank K. Podgorski and A. Hu (Janelia Research Campus) for advice on tissue-damage assessment. We also thank H.G. Chae and D. Florin Albeanu (Cold Spring Harbor Laboratory) for sharing the head-bar and holder design for awake imaging. Last, we thank the Warden lab (Cornell University) for providing the microtome for brain sectioning. The project was supported by DARPA (W911NF-14-1-0012 to C.X.; HR0011-16-2-0017 to M.J.S.), NIH/NINDS (U01NS090530 to C.X.), the National Science Foundation (NeuroNex Grant DBI-1707312 to C.X.), and the Intelligence Advanced Research Projects Activity (IARPA) via Department of Interior/Interior Business Center (DoI/IBC) (contract number D16PC00003 to C.X.). The US Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright annotation thereon. Disclaimer: The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of IARPA, DoI/IBC, or the US Government.
Author information
Authors and Affiliations
Contributions
C.X. conceived the study and supervised the project. T.W., D.G.O., and N.G.H. designed and performed the experiments. C.W. set up awake imaging and performed immunohistology. C.-H.W., B.Z., and Y.Z. tested skull preparations for improving the optical transparency of the cranium for long-term imaging. C.X. and M.J.S. supervised the study. T.W. analyzed the data. T.W. and C.X. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Visual examination of skull transparency after skull preparation.
The bone surface was covered by Loctite 4305, and a #1 round coverslip (5 mm in diameter) was laid on top of it. The glue was cured by UV light. Day 1 was the day of the skull preparation. The images were taken under a stereoscope. Photos on day 7 and day 14 were taken after 2 and 3 recording sessions, respectively. Scale bar, 5 mm.
Supplementary Figure 2 Comparison of 1,320-nm 3PM, 1,320-nm 2PM, and 920-nm 2PM for vasculature imaging.
a, Vasculature imaging of a wild-type mouse (C57BL/6 J, male, 12-week-old, similar results n = 3) in the same cortical column under the central area of the parietal bone. Each site was imaged by 920-nm 2PE of fluorescein, 1320-nm 3PE of fluorescein, and 1320-nm 2PE of Alexa 680 during the same imaging session. The number in the upper right corner of each frame indicates the average power used in unit of mW. The 920-nm excitation source was operated at 80 MHz repetition rate, and 1320-nm at 400 kHz. Each frame was integrated for 50 s to ensure high signal-to-noise ratio. For the images in the row labeled as “1320-nm 2PE Alexa 680 Gamma = 1.5”, each pixel value S is raised to S3/2 to show that the lower contrast in 2PM images cannot be compensated by nonlinear contrast stretching as post-processing. For fair visual comparison between the different imaging modalities, all image contrasts are linearly stretched to saturate the brightest 0.2% pixels. Scale bar, 30 μm. b, Comparison of 920-nm 2PM and 1320-nm 3PM through-skull imaging of neurons in a transgenic mouse (CamKII-tTA/tetO-GCaMP6s, female, 10-week-old, n = 1) at 150 μm cortical depth with ~ 105 μm skull thickness. Each frame was integrated for 40 s. Scale bar, 20 μm.
Supplementary Figure 3 Raw data of through-skull neuronal activity traces.
The unprocessed version of a subset of the activity traces in Fig. 2f, representing neurons of different size, brightness, and activity pattern (similar results n = 5). The signal of each neuron (in photons/neuron/frame) was plotted against time, at the original 8.49 Hz recording frame rate, without any temporal down-sampling or low-pass filtering. Fluorescence intensity was converted to photon counts per frame per neuron to confirm that the baseline fluctuations are mostly due to photon shot noise. Above each trace, the value of the measured mean (F0) and variance (VarF0) of the baseline fluorescence photon-count rate, and the areas of each neuron, are indicated. For each trace, its processed version and the corresponding neuron can be found in Figs. 2e and 2f respectively, by its index to the left of the trace.
Supplementary Figure 4 Through-skull recording of neuronal activity in deeper cortex in an awake animal.
Spontaneous activity traces from deeper cortical neurons recorded in a GCaMP6s mouse under awake condition (CamKII-tTA/tetO-GCaMP6s, female, 8–9 weeks old, n = 1). In each panel, the high resolution image on the left shows the recording site (green: GCaMP6s fluorescence; red: THG; Scale bar, 50 μm). The neurons are indexed for their corresponding time traces. The day since the skull preparation, the age of the mouse, the average power, the repetition rate, the imaging depth, and the size of FOV are indicated at the top of each image. The right panels show activity traces of the neurons, recorded at 8.49 Hz frame rate. The traces were normalized to their baselines and low-pass filtered by a hamming window with 1.06 s time constant. The thickness of the skull was about 120 μm. a, A recording site located at 375 μm cortical depth observed in two imaging sessions 11 days apart (similar results n = 3). We leveraged the THG channel to visualize the vasculature map, which serves as the landmark for locating the same recording site. Due to small variations in each imaging session (for example, mouse orientation, tilting, the finite focal depth, etc.), however, it is difficult to match the recording site exactly. Nonetheless, some neurons can be identified in both imaging sessions. These neurons are indexed alphabetically, and the same labels are used for both imaging sessions. Neurons only observed in one of the two imaging sessions are indexed numerically. b, A recording site located at 430 μm cortical depth (n = 1). An additional image without any neuron indexing (unlabeled) is shown for better visualization of the image. c, The deepest recording site in this study located at 465 μm cortical depth (n = 1).
Supplementary Figure 5 Longitudinal through-skull recording of neuronal activity at the same locations in an awake animal over 4 weeks.
Spontaneous activity traces from cortical L2/3 neurons were recorded in a GCaMP6s mouse under awake condition (CamKII-tTA/tetO-GCaMP6s, female, 8–11 weeks old, and similar results n = 3) over a period of 4 weeks after the skull preparation. The left panels show high resolution images of a recording site at 240 μm cortical depth, and the right panels show another site at 275 μm depth (the same site as in Figs. 2e and 2f). Green: GCaMP6s fluorescence; red: THG; Scale bar, 50 μm. THG visualizes the vasculature map, which was used to navigate to approximately the same location for recording. As discussed in Supplementary Fig. 4, it is difficult to match the recording site exactly for each imaging session. Therefore, only a fraction of the neurons can be identified in multiple imaging sessions. These neurons are indexed alphabetically, and the same unique letter for each neuron is used in all imaging sessions. The day since the skull preparation, the age of the mouse, the average power used for activity recording, the imaging depth, and the size of FOV are indicated at the top of each image. 8 recording sessions were performed at each site (4 at each site are shown). The middle panels show the activity traces of the indexed neurons, recorded at 8.49 Hz frame rate, and each was normalized to its baseline and low-pass filtered by a hamming window with 1.06 s time constant. The repetition rate for all imaging was 800 kHz. The thickness of the skull was about 120 μm.
Supplementary Figure 6 Immunolabeled coronal sections of mouse brains after through-skull imaging at 1,320 nm.
The brain was imaged by 1320-nm 3PM operating at 400 kHz repetition rate. The imaging depth was about 200 μm under the cortical surface, and the scanned area was 200 × 200 μm, at the location of ~ 2.5 mm lateral and 1 mm caudal to the bregma point. The illumination continued for 30 minutes, and the brain was fixed after 16 h (n = 1). Scale bar, 1 mm.
Supplementary Figure 7 Through-skull imaging of mouse vasculature with 1,700-nm 3PM.
a, Through-skull imaging of Texas Red labeled vasculature of mouse brain with an acute preparation using water to fill the space between the cover glass and the skull (red, fluorescence; violet, THG, similar results n = 3). The skull-brain interface is indicated by the boundary between the fluorescence and the bright THG signal. b, THG images of osteocytes within the mouse skull (n = 3). c, Comparison of through-skull (TS) and post-craniotomy (PC) frames for the same mouse and FOV at two depths below the brain surface (n = 1). d, Signal attenuation before and after craniotomy (n = 1). The plot displays THG signal attenuation within the skull (black dots), fluorescence signal attenuation within the brain below the skull (blue dots), and fluorescence signal attenuation in the same region of the brain post-craniotomy (green dots). The curves were matched at depth = 0 (that is, the skull-brain interface). The attenuation length of the brain, through-skull, was 240 μm, and the attenuation length post-craniotomy was 345 μm. Scale bars, 50 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Notes 1 and 2
Supplementary Protocol
Protocol for skull preparation for chronic imaging
Supplementary Video 1
Unprocessed video of longitudinal through-skull three-photon calcium imaging at real-time speed. The video contains three excerpts of through-skull three-photon calcium imaging on days 3, 12, and 28 after the skull preparation. The calcium activity movie is completely unprocessed and looks exactly the same as during an imaging session.
Supplementary Video 2
Time-lapse video of longitudinal through-skull three-photon calcium imaging. The video contains three excerpts of through-skull three-photon calcium imaging on days 3, 14, and 28 after the skull preparation. The calcium activity movie has been averaged, de-noised, and accelerated, as described in the Methods.
Rights and permissions
About this article
Cite this article
Wang, T., Ouzounov, D.G., Wu, C. et al. Three-photon imaging of mouse brain structure and function through the intact skull. Nat Methods 15, 789–792 (2018). https://doi.org/10.1038/s41592-018-0115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-018-0115-y
This article is cited by
-
Photostimulation of brain lymphatics in male newborn and adult rodents for therapy of intraventricular hemorrhage
Nature Communications (2023)
-
A three-photon head-mounted microscope for imaging all layers of visual cortex in freely moving mice
Nature Methods (2023)
-
Miniature three-photon microscopy maximized for scattered fluorescence collection
Nature Methods (2023)
-
Computational conjugate adaptive optics microscopy for longitudinal through-skull imaging of cortical myelin
Nature Communications (2023)
-
Near-infrared fluorescence lifetime imaging of amyloid-β aggregates and tau fibrils through the intact skull of mice
Nature Biomedical Engineering (2023)