Abstract
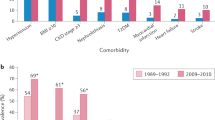
Gout is the most common form of inflammatory arthritis worldwide and is characterized by painful recurrent flares of inflammatory arthritis that are associated with a transiently increased risk of adverse cardiovascular events. Furthermore, gout is associated with multiple cardiometabolic–renal comorbidities such as type 2 diabetes, chronic kidney disease and cardiovascular disease. These comorbidities, potentially combined with gout flare-related inflammation, contribute to persistent premature mortality in gout, independently of serum urate concentrations and traditional cardiovascular risk factors. Although better implementation of standard gout care could improve gout outcomes, deliberate efforts to address the cardiovascular risk in patients with gout are likely to be required to reduce mortality. Sodium–glucose cotransporter type 2 (SGLT2) inhibitors are approved for multiple indications owing to their ability to lower the risk of all-cause and cardiovascular death, hospitalizations for heart failure and chronic kidney disease progression, making them an attractive treatment option for gout. These medications have also been shown to lower serum urate concentrations, the causal culprit in gout risk, and are associated with a reduced risk of incident and recurrent gout, potentially owing to their purported anti-inflammatory effects. Thus, SGLT2 inhibition could simultaneously address both the symptoms of gout and its comorbidities.
Key points
-
Sodium-glucose cotransporter type 2 (SGLT2) inhibitors have revolutionized the management of type 2 diabetes, heart failure and chronic kidney disease and have been incorporated into multiple subspecialty management guidelines.
-
SGLT2 inhibitors hold promise as an attractive multi-purpose treatment option for patients with gout to simultaneously address cardiometabolic–renal comorbidities and gout-related morbidity.
-
SGLT2 inhibitors have been shown to lower serum urate concentrations and the risk of incident and recurrent gout flares without apparently increasing the risk of paradoxical gout flares.
-
The exact mechanisms underlying the urate-lowering and anti-gout effects of SGLT2 inhibitors remain under active investigation but might involve enhanced uricosuria and anti-inflammatory pathways.
-
Although additional research is required to determine the role of SGLT2 inhibitors in gout management, available evidence suggests that these drugs have the potential to improve outcomes among patients with gout.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Choi, H. K., Mount, D. B. & Reginato, A. M. Pathogenesis of gout. Ann. Intern. Med. 143, 499–516 (2005).
Yokose, C. et al. Trends in prevalence of gout among US Asian adults, 2011–2018. JAMA Netw. Open 6, e239501 (2023).
Xia, Y. et al. Global, regional and national burden of gout, 1990–2017: a systematic analysis of the Global Burden of Disease Study. Rheumatology 59, 1529–1538 (2020).
Elfishawi, M. M. et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J. Rheumatol. 45, 574–579 (2018).
Safiri, S. et al. Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990–2017: a systematic analysis of the global burden of disease study 2017. Arthritis Rheumatol. 72, 1916–1927 (2020).
Dehlin, M., Jacobsson, L. & Roddy, E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 16, 380–390 (2020).
Edwards, N. L. Quality of care in patients with gout: why is management suboptimal and what can be done about it? Curr. Rheumatol. Rep. 13, 154–159 (2011).
Sarawate, C. A. et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin. Proc. 81, 925–934 (2006).
Neogi, T., Hunter, D. J., Chaisson, C. E., Allensworth-Davies, D. & Zhang, Y. Frequency and predictors of inappropriate management of recurrent gout attacks in a longitudinal study. J. Rheumatol. 33, 104–109 (2006).
Chock, Y. P., Ross, J. S., Suter, L. G. & Rhee, T. G. Gout treatment in the USA from 2009 to 2016: a repeated cross-sectional analysis. J. Gen. Intern. Med. 36, 1134–1136 (2021).
Singh, J. A. & Cleveland, J. D. Time trends in opioid use disorder hospitalizations in gout, rheumatoid arthritis, fibromyalgia, osteoarthritis, and low back pain. J. Rheumatol. 48, 775–784 (2021).
Dalal, D. S. et al. Prescription opioid use among patients with acute gout discharged from the emergency department. Arthritis care Res. 72, 1163–1168 (2020).
Jinno, S., Hasegawa, K., Neogi, T., Goto, T. & Dubreuil, M. Trends in emergency department visits and charges for gout in the United States between 2006 and 2012. J. Rheumatol. 43, 1589–1592 (2016).
Garg, R. et al. Gout-related health care utilization in US emergency departments, 2006 through 2008. Arthritis care Res. 65, 571–577 (2013).
Lim, S. Y. et al. Trends in gout and rheumatoid arthritis hospitalizations in the United States, 1993–2011. J. Am. Med. Assoc. 315, 2345–2347 (2016).
Rai, S. K. et al. Trends in gout and rheumatoid arthritis hospitalizations in Canada From 2000 to 2011. Arthritis care Res. 69, 758–762 (2017).
Russell, M. D. et al. Rising incidence of acute hospital admissions due to gout. J. Rheumatol. 47, 619–623 (2020).
Dehlin, M. & Jacobsson, L. T. H. Trends in gout hospitalization in Sweden. J. Rheumatol. 45, 145–146 (2018).
Cipolletta, E. et al. Association Between gout flare and subsequent cardiovascular events among patients with gout. J. Am. Med. Assoc. 328, 440–450 (2022).
Choi, H. K. & McCormick, N. Beyond joint pain, could each gout flare lead to heart attack? Nat. Rev. Rheumatol. 18, 619–620 (2022).
Zhu, Y., Pandya, B. J. & Choi, H. K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am. J. Med. 125, 679–687.e1 (2012).
Kuo, C. F., Grainge, M. J., Mallen, C., Zhang, W. & Doherty, M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann. Rheum. Dis. 75, 210–217 (2016).
Choi, H. K. & Curhan, G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 116, 894–900 (2007).
Abbott, R. D., Brand, F. N., Kannel, W. B. & Castelli, W. P. Gout and coronary heart disease: the Framingham Study. J. Clin. Epidemiol. 41, 237–242 (1988).
Gupta, S. et al. The risk of cardiovascular disease among male and female participants treated for gout in the Multi-Ethnic Study of Atherosclerosis (MESA). Gout Urate Cryst. Depos. Dis. 1, 89–98 (2023).
Bardin, T., Letavernier, E. & Correas, J.-M. The gouty kidney: a reappraisal. Gout Urate Cryst. Depos. Dis. 1, 25–36 (2023).
Disveld, I. J. M. et al. Crystal-proven gout patients have an increased mortality due to cardiovascular diseases, cancer, and infectious diseases especially when having tophi and/or high serum uric acid levels: a prospective cohort study. Clin. Rheumatol. 38, 1385–1391 (2019).
Kuo, C. F. et al. Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology 49, 141–146 (2010).
Fisher, M. C., Rai, S. K., Lu, N., Zhang, Y. & Choi, H. K. The unclosing premature mortality gap in gout: a general population-based study. Ann. Rheum. Dis. 76, 1289–1294 (2017).
Marty-Ane, A. et al. Crystal deposition measured with dual-energy computed tomography: association with mortality and cardiovascular risks in gout. Rheumatology 60, 4855–4860 (2021).
McCormick, N. et al. Persistent premature mortality in gout: nationwide prospective cohort study [Abstract]. Ann. Rheum. Dis. 82, 436 (2023).
FitzGerald, J. D. et al. American College of Rheumatology guideline for the management of gout. Arthritis Care Res. 72, 744–760 (2020).
Richette, P. et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 76, 29–42 (2017).
Doherty, M. et al. Nurse-led care versus general practitioner care of people with gout: a UK community-based randomised controlled trial. Ann. Rheum. Dis. https://doi.org/10.2139/ssrn.3221414 (2018).
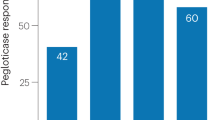
Becker, M. A., Schumacher, H. R., MacDonald, P. A., Lloyd, E. & Lademacher, C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J. Rheumatol. 36, 1273–1282 (2009).
Schumacher, H. R. Jr, Becker, M. A., Lloyd, E., MacDonald, P. A. & Lademacher, C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology 48, 188–194 (2009).
Doherty, M. et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 392, 1403–1412 (2018).
Choi, H. K., McCormick, N. & Yokose, C. Excess comorbidities in gout: the causal paradigm and pleiotropic approaches to care. Nat. Rev. Rheumatol. 18, 97–111 (2022).
Mackenzie, I. S. et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 396, 1745–1757 (2020).
Badve, S. V. et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 382, 2504–2513 (2020).
Doria, A. et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 382, 2493–2503 (2020).
US Food and Drug Administration. FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat). https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat (21 February 2019).
McMullan, C. J., Borgi, L., Fisher, N., Curhan, G. & Forman, J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: a randomized controlled trial. Clin. J. Am. Soc. Nephrol. 12, 807–816 (2017).
Gaffo, A. L. et al. Effect of serum urate lowering with allopurinol on blood pressure in young adults: a randomized, controlled, crossover trial. Arthritis Rheumatol. 73, 1514–1522 (2021).
White, W. B. et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 378, 1200–1210 (2018).
Hare, J. M. et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 51, 2301–2309 (2008).
Givertz, M. M. et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation 131, 1763–1771 (2015).
Scheen, A. J. Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 16, 556–577 (2020).
Khunti, K. SGLT2 inhibitors in people with and without T2DM. Nat. Rev. Endocrinol. 17, 75–76 (2021).
ElSayed, N. A. et al. 9. pharmacologic approaches to glycemic treatment: standards of care in diabetes — 2023. Diabetes Care 46, S140–S157 (2023).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41, 2669–2701 (2018).
Kidney Disease: Improving Global Outcomes (KDGIO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 102, S1–S127 (2022).
de Boer, I. H. et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 45, 3075–3090 (2022).
Heidenreich, P. A. et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, e263–e421 (2022).
Arnett, D. K. et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, e177–e232 (2019).
US Food and Drug Administration. Highlights of Prescribing Information — Invokana. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/204042s040lbl.pdf (2023).
US Food and Drug Administration. Highlights of Prescribing Information — Farxiga. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/202293s026lbl.pdf (2023).
US Food and Drug Administration. Highlights of Prescribing Information — Jardiance. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/204629s040lbl.pdf (2023).
Zhao, Y. et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 20, 458–462 (2018).
Yip, A. S. Y. et al. Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on serum urate levels in patients with and without diabetes: a systematic review and meta-regression of 43 randomized controlled trials. Ther. Adv. Chronic Dis. 13, 20406223221083509 (2022).
Hu, X. et al. Effects of sodium-glucose cotransporter 2 inhibitors on serum uric acid in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Diabetes Obes. Metab. 24, 228–238 (2022).
Xin, Y. et al. Effects of sodium glucose cotransporter-2 inhibitors on serum uric acid in type 2 diabetes mellitus: a systematic review with an indirect comparison meta-analysis. Saudi J. Biol. Sci. 26, 421–426 (2019).
Doehner, W. et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR-reduced trial. Eur. Heart J. 43, 3435–3446 (2022).
Packer, M. et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 383, 1413–1424 (2020).
McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008 (2019).
McDowell, K. et al. Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF. Eur. J. Heart Fail. 24, 1066–1076 (2022).
Ferreira, J. P. et al. Empagliflozin and uric acid metabolism in diabetes: a post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes. Metab. 24, 135–141 (2022).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Li, J. et al. The effects of canagliflozin on gout in type 2 diabetes: a post-hoc analysis of the CANVAS Program. Lancet Rheumatol. 1, e220–e228 (2019).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Stack, A. G. et al. Dapagliflozin added to verinurad plus febuxostat further reduces serum uric acid in hyperuricemia: the QUARTZ Study. J. Clin. Endocrinol. Metab. 106, e2347–e2356 (2021).
Yokose C et al. Serum urate change among gout patients initiating sodium-glucose cotransporter type 2 inhibitors (SGLT2i) vs. sulfonylureas: a comparative effectiveness analysis [Abstract]. Arthritis Rheumatol. 2023;75.
Vargas-Santos AB, Peloquin C, Kim SC, Neogi T. Sodium-glucose co-transporter-2 inhibitors and the risk for gout — a comparison among canagliflozin, dapagliflozin and empagliflozin [Abstract]. Arthritis Rheumatol. 2020;72.
Butt, J. H. et al. Association of dapagliflozin use with clinical outcomes and the introduction of uric acid-lowering therapy and colchicine in patients with heart failure with and without gout: a patient-level pooled meta-analysis of DAPA-HF and DELIVER. JAMA Cardiol. 8, 386–393 (2023).
Neogi, T. et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 74, 1789–1798 (2015).
Gaffo, A. L. et al. Brief Report: validation of a definition of flare in patients with established gout. Arthritis Rheumatol. 70, 462–467 (2018).
Fralick, M., Chen, S. K., Patorno, E. & Kim, S. C. Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: a population-based cohort study. Ann. Intern. Med. 172, 186–194 (2020).
Lund, L. C., Hojlund, M., Henriksen, D. P., Hallas, J. & Kristensen, K. B. Sodium-glucose cotransporter-2 inhibitors and the risk of gout: a Danish population based cohort study and symmetry analysis. Pharmacoepidemiol. Drug. Saf. 30, 1391–1395 (2021).
Chung, M. C. et al. Association of sodium-glucose transport protein 2 inhibitor use for type 2 diabetes and incidence of gout in Taiwan. JAMA Netw. Open 4, e2135353 (2021).
Zhou, J. et al. Lower risk of gout in sodium glucose cotransporter 2 (SGLT2) inhibitors versus dipeptidyl peptidase-4 (DPP4) inhibitors in type-2 diabetes. Rheumatology 62, 1501–1510 (2023).
Yokose, C. et al. Risk of incident gout associated with initiation of sodium-glucose cotransporter-2 inhibitors versus other second-line agents among metformin users with type 2 diabetes [Abstract]. Ann. Rheum. Dis. 2023:171.
Banerjee, M., Pal, R. & Mukhopadhyay, S. Can SGLT2 inhibitors prevent incident gout? A systematic review and meta-analysis. Acta Diabetol. 59, 783–791 (2022).
Bailey, C. J. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes. Metab. 21, 1291–1298 (2019).
Qaseem, A., Harris, R. P. & Forciea, M. A. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 166, 58–68 (2017).
McCormick, N. et al. Comparative effectiveness of sodium-glucose cotransporter-2 inhibitors for recurrent gout flares and gout-primary emergency department visits and hospitalizations : a general population cohort study. Ann. Intern. Med. 176, 1067–1080 (2023).
Choi, H. K., Zhang, Y. & Dalbeth, N. When underlying biology threatens the randomization principle - initial gout flares of urate-lowering therapy. Nat. Rev. Rheumatol. 18, 543–549 (2022).
Wei, J. et al. Gout flares and mortality after sodium-glucose cotransporter-2 inhibitor treatment for gout and type 2 diabetes. JAMA Netw. Open 6, e2330885 (2023).
Cowie, M. R. & Fisher, M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 17, 761–772 (2020).
Zannad, F. et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur. Heart J. 43, 4991–5002 (2022).
Ahmadieh, H. & Azar, S. Effects of sodium glucose cotransporter-2 inhibitors on serum uric acid in type 2 diabetes mellitus. Diabetes Technol. Ther. 19, 507–512 (2017).
Vallon, V. & Thomson, S. C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 16, 317–336 (2020).
Nespoux, J. & Vallon, V. Renal effects of SGLT2 inhibitors: an update. Curr. Opin. Nephrol. Hypertens. 29, 190–198 (2020).
Dalbeth, N. et al. Gout. Nat. Rev. Dis. Primers 5, 69 (2019).
Novikov, A. et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am. J. Physiol. Renal Physiol. 316, F173–F185 (2019).
Quinones Galvan, A. et al. Effect of insulin on uric acid excretion in humans. Am. J. Physiol. 268, E1–E5 (1995).
Cherney, D. Z., Odutayo, A., Aronson, R., Ezekowitz, J. & Parker, J. D. Sodium glucose cotransporter-2 inhibition and cardiorenal protection: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 74, 2511–2524 (2019).
Muscelli, E. et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am. J. Hypertens. 9, 746–752 (1996).
Ter Maaten, J. C. et al. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin. Sci. 92, 51–58 (1997).
Facchini, F., Chen, Y. D., Hollenbeck, C. B. & Reaven, G. M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. J. Am. Med. Assoc. 266, 3008–3011 (1991).
McCormick, N. et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional mendelian randomization. Arthritis Rheumatol. 73, 2096–2104 (2021).
Elrakaybi, A., Laubner, K., Zhou, Q., Hug, M. J. & Seufert, J. Cardiovascular protection by SGLT2 inhibitors — do anti-inflammatory mechanisms play a role? Mol. Metab. 64, 101549 (2022).
Mancini, S. J. et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci. Rep. 8, 5276 (2018).
Maayah, Z. H., Ferdaoussi, M., Takahara, S., Soni, S. & Dyck, J. R. B. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology 29, 269–279 (2021).
Hawley, S. A. et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 65, 2784–2794 (2016).
Xu, J., Kitada, M., Ogura, Y., Liu, H. & Koya, D. Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells 10, 1457 (2021).
Theofilis, P. et al. The impact of SGLT2 inhibitors on inflammation: a systematic review and meta-analysis of studies in rodents. Int. Immunopharmacol. 111, 109080 (2022).
Scisciola, L. et al. Anti-inflammatory role of SGLT2 inhibitors as part of their anti-atherosclerotic activity: data from basic science and clinical trials. Front. Cardiovasc. Med. 9, 1008922 (2022).
Heerspink, H. J. L. et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 62, 1154–1166 (2019).
Byrne, N. J. et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) inflammasome activation in heart failure. Circ. Heart Fail. 13, e006277 (2020).
Kim, S. R. et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 11, 2127 (2020).
Banerjee, M., Pal, R., Maisnam, I., Chowdhury, S. & Mukhopadhyay, S. Serum uric acid lowering and effects of sodium-glucose cotransporter-2 inhibitors on gout: a meta-analysis and meta-regression of randomized controlled trials. Diabetes Obes. Metab. 25, 2697–2703 (2023).
Zinman, B., Lachin, J. M. & Inzucchi, S. E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 374, 1094 (2016).
Wanner, C., Inzucchi, S. E. & Zinman, B. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 1801–1802 (2016).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Group, E.-K. C. et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2022).
Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385, 1451–1461 (2021).
Solomon, S. D. et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387, 1089–1098 (2022).
European Medicines Agency. Jardiance : EPAR - Product Information. https://www.ema.europa.eu/en/medicines/human/EPAR/jardiance#ema-inpage-item-product-info (accessed 29 January 2024).
European Medicines Agency. Forxiga. https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga (accessed 29 January 2024).
European Medicines Agency. Invokana. https://www.ema.europa.eu/en/medicines/human/EPAR/invokana (accessed 29 January 2024).
Roughley, M. J., Belcher, J., Mallen, C. D. & Roddy, E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res. Ther. 17, 90 (2015).
Liu, J. et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci. Rep. 7, 2824 (2017).
Gomez-Peralta, F. et al. Practical approach to initiating SGLT2 inhibitors in type 2 diabetes. Diabetes Ther. 8, 953–962 (2017).
Nauck, M. A. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug. Des. Devel Ther. 8, 1335–1380 (2014).
Vardeny, O. & Vaduganathan, M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 7, 169–172 (2019).
Scheen, A. J. An update on the safety of SGLT2 inhibitors. Expert. Opin. Drug. Saf. 18, 295–311 (2019).
Banerjee, M., Maisnam, I., Pal, R. & Mukhopadhyay, S. Mineralocorticoid receptor antagonists with sodium-glucose co-transporter-2 inhibitors in heart failure: a meta-analysis. Eur. Heart J. 44, 3686–3696 (2023).
Chan, Y. H. et al. Association of acute increases in serum creatinine with subsequent outcomes in patients with type 2 diabetes mellitus treated with sodium-glucose cotransporter 2 inhibitor or dipeptidyl peptidase-4 inhibitor. Eur. Heart J. Qual. Care Clin. Outcomes 9, 397–407 (2023).
US Food and Drug Administration. FDA drug safety communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-confirms-increased-risk-leg-and-foot-amputations-diabetes-medicine (accessed 4 May 2023).
US Food and Drug Administration. FDA drug safety communication: FDA removes Boxed Warning about risk of leg and foot amputations for the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-removes-boxed-warning-about-risk-leg-and-foot-amputations-diabetes-medicine-canagliflozin (accessed 4 May 4 2023).
Rashid, N. et al. Patient and clinical characteristics associated with gout flares in an integrated healthcare system. Rheumatol. Int. 35, 1799–1807 (2015).
Adhikari, R. et al. National trends in use of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists by cardiologists and other specialties, 2015 to 2020. J. Am. Heart Assoc. 11, e023811 (2022).
Doehner, W. & Packer, M. Sodium-glucose cotransporter 2 inhibitor treatment lowers serum uric acid in patients with heart failure with reduced ejection fraction — lessons from clinical trials. Letter regarding the article ‘Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF’. Eur. J. Heart Fail. 24, 1993–1994 (2022).
Acknowledgements
The authors would like to thank S. Tanikella for assistance in generating the figures included in this manuscript.
Author information
Authors and Affiliations
Contributions
C.Y. and N.M. researched data for the article. C.Y. wrote the article. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.D. has received consulting fees, speaker fees or grants from Arthrosi, AstraZeneca, Dyve Biosciences, Hikma, Horizon, JPI, JW Pharmaceutical Corporation, LG Chem, Novartis, PK Med, Protalix, PTC Therapeutics, Selecta and Unlocked Labs, outside the submitted work. F.L. has received consulting fees from Horizon Biosciences, Mayoly Spindler, Novartis, Olatec and SOBI-Selecta, and unrestricted grants for the European Crystal Network workshops from Arthrosi, AstraZeneca, Dyve Biosciences, Horizon Biosciences, Mayoly Spindler and Olatec, outside the submitted work. A.G has served as consultant SOBI, PK Med and serves on a data monitoring committee for Atom Bioscience. J.L.J. is a Trustee of the American College of Cardiology and a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, HeartFlow, Innolife and Novartis; has received consulting income from Abbott Diagnostics, AstraZeneca, Beckman Coulter, Jana Care, Janssen, Novartis, Prevencio, Quidel and Roche Diagnostics; and participates in clinical end point committees/data safety monitoring boards for AbbVie, Abbott, Bayer, Siemens, Pfizer and Takeda, outside the submitted work. R.T. has served or serves as a consultant for Acquist Therapeutics, Allena, AstraZeneca, Atom Bioscience, Fortress/Urica, Generate Biomedicines, Horizon Therapeutics, LG Chem, Selecta Biosciences and Synlogic, and was a previous recipient of a research grant from AstraZeneca. R.T. serves as the non-salaried President of the G-CAN (Gout, Hyperuricemia and Crystal-Associated Disease Network) research society; over its 9 years of existence, G-CAN annually has received unrestricted arms-length grant support from pharma donors. D.J.W. serves on a data monitoring committee for Novo Nordisk, outside the submitted work. H.K.C. reports research support from Ironwood and Horizon, and consulting fees from Horizon, Ironwood, Kowa, Selecta, Takeda and Vaxart. M.E.S. receives research funding from AbbVie, Angion, Cabaletta, EMD-Serono, Gilead, Novartis, Otsuka and Roche; serves on scientific advisory boards for Calliditas, Mallinckrodt, Novartis, Travere and Vera; and serves as a DSMB member for Alpine Immunosciences. T.P. has received consulting and speaker fees from Novartis and consulting and research grants from Horizon Pharmaceuticals. C.Y., J.F., N.M. and A.A. declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Mainak Banerjee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yokose, C., McCormick, N., Abhishek, A. et al. The clinical benefits of sodium–glucose cotransporter type 2 inhibitors in people with gout. Nat Rev Rheumatol 20, 216–231 (2024). https://doi.org/10.1038/s41584-024-01092-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01092-x