Abstract
Xanthine oxidase inhibitors such as allopurinol and febuxostat have been the mainstay urate-lowering therapy (ULT) for treating hyperuricaemia in patients with gout. However, not all patients receiving oral ULT achieve the target serum urate level, in part because some patients cannot tolerate, or have actual or misconceived contraindications to, their use, mainly due to comorbidities. ULT dosage is also limited by formularies and clinical inertia. This failure to sufficiently lower serum urate levels can lead to difficult-to-treat or uncontrolled gout, usually due to poorly managed and/or under-treated gout. In species other than humans, uricase (urate oxidase) converts urate to allantoin, which is more soluble in urine than uric acid. Exogenic uricases are an exciting therapeutic option for patients with gout. They can be viewed as enzyme replacement therapy. Uricases are being used to treat uncontrolled gout, and can achieve rapid reduction of hyperuricaemia, dramatic resolution of tophi, decreased chronic joint pain and improved quality of life. Availability, cost and uricase immunogenicity have limited their use. Uricases could become a leading choice in severe and difficult-to-treat gout as induction and/or debulking therapy (that is, for lowering of the urate pool) to be followed by chronic oral ULT. This Review summarizes the evidence regarding available uricases and those in the pipeline, their debulking effect and their outcomes related to gout and beyond.
Key points
-
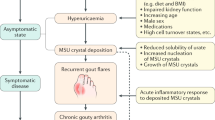
The lack of a functional uricase to metabolize uric acid and facilitate its elimination renders humans susceptible to hyperuricaemia and gout.
-
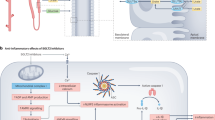
Uricases are foreign to the human immune system; thus, treatment with exogenous uricases can lead to the development of anti-drug antibodies, which can decrease the efficacy of uricases and increase the risk of infusion reactions.
-
Combination therapy of uricases with immunosuppressants decreases the incidence of anti-drug antibodies and increases the proportion of patients who respond to uricase treatment.
-
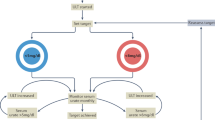
Screening for glucose-6-phosphate dehydrogenase deficiency before the first infusion of uricase is crucial.
-
Implementation of a ‘stopping rule’ for uricase treatment based on two sequential pre-infusion measurements of serum urate levels >6.0 mg/dl reduces the risk of infusion reactions.
-
Two uricases, rasburicase and pegloticase, are currently available, and several other uricases are in the drug development pipeline.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ruoff, G. & Edwards, N. L. Overview of serum uric acid treatment targets in gout: why less than 6 mg/dL? Postgrad. Med. 128, 706–715 (2016).
Schlesinger, N., Watson, D. J. & Norquist, J. M. Serum urate during acute gout. J. Rheumatol. 36, 1287–1289 (2009).
Terkeltaub, R., Bushinsky, D. A. & Becker, M. A. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res. Ther. 8, S4 (2006).
Johnson, R. J., Titte, S., Cade, J. R., Rideout, B. A. & Oliver, W. J. Uric acid, evolution and primitive cultures. Semin. Nephrol. 25, 3–8 (2005).
Álvarez-Lario, B. & Macarrón-Vicente, J. Uric acid and evolution. Rheumatology 49, 2010–2015 (2010).
Kratzer, J. T. et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl Acad. Sci. USA 111, 3763–3768 (2014).
Rashid, N. et al. Modifiable factors associated with allopurinol adherence and outcomes among patients with gout in an integrated healthcare system. J. Rheumatol. 42, 504–512 (2015).
FitzGerald, J. D. et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res. 72, 744–760 (2020).
Richette, P. et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 76, 29–42 (2017).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Perez-Ruiz, F., Calabozo, M., Pijoan, J. I., Herrero-Beites, A. M. & Ruibal, A. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum. 47, 356–360 (2002).
Perez-Ruiz, F., Martin, I. & Canteli, B. J. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J. Rheumatol. 34, 1888–1893 (2007).
Ebstein, E. et al. Ultrasound evaluation in follow-up of urate-lowering therapy in gout: the USEFUL study. Rheumatology 58, 410–417 (2019).
Chui, C. S. K. et al. Volumetric reduction and dissolution prediction of monosodium urate crystal during urate-lowering therapy – a study using dual-energy computed tomography. Mod. Rheumatol. 31, 875–884 (2021).
Choi, H. K. et al. Dual energy computed tomography in tophacheous gout. Ann. Rheum. Dis. 68, 1609–1612 (2009).
Dalbeth, N. et al. Urate crystal deposition in asymptomatic hyperuricemia and symptomatic gout: a dual energy CT study. Ann. Rheum. Dis. 74, 908–911 (2015).
Schlesinger, N. & Thiele, R. G. The pathogenesis of bone erosions in gouty arthritis. Ann. Rheum. Dis. 69, 1907–1912 (2010).
Dalbeth, N. et al. Relationship between structural joint damage and urate deposition in gout: a plain radiography and dual-energy CT study. Ann. Rheum. Dis. 74, 1030–1036 (2015).
Shi, D. et al. Relationship between urate within tophus and bone erosion according to the anatomic location of urate deposition in gout: a quantitative analysis using dual-energy CT volume measurements. Medicine 98, e18431 (2019).
Dalbeth, N. et al. Development of a computed tomography method of scoring bone erosion in patients with gout: validation and clinical implications. Rheumatology 50, 410–416 (2011).
Araujo, E. G. et al. Tophus resolution with pegloticase: a prospective dual-energy CT study. RMD Open 1, e000075 (2015).
Modjinou, D. V. et al. Comparison of dual-energy CT, ultrasound and surface measurement for assessing tophus dissolution during rapid urate debulking. Clin. Rheumatol. 36, 2101–2107 (2017).
Dalbeth, N. et al. Dual-energy CT assessment of rapid monosodium urate depletion and bone erosion remodeling during pegloticase plus methotrexate co-therapy. Rheumatology 61, 4898–4904 (2022).
Mandell, B. F., Yeo, A. E. & Lipsky, P. E. Tophus resolution in patients with chronic refractory gout who have persistent urate-lowering responses to pegloticase. Arthritis Res. Ther. 20, 286 (2018).
Hui, M. et al. The British Society for Rheumatology guideline for the management of gout. Rheumatology 56, e1–e20 (2017).
Neilson, J., Bonnon, A., Dickson, A., Roddy, E. & Guideline Committee. Gout: diagnosis and management – summary of NICE guidance. BMJ 378, o1754 (2022).
Garay, R. P., El-Gewely, M. R., Labaune, J. P. & Richette, P. Therapeutic perspectives on uricases for gout. Joint Bone Spine 79, 237–242 (2012).
Lipsky, P. E. et al. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res. Ther. 16, R60 (2014).
Sundy, J. S. et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 306, 711–720 (2011).
FDA. Highlights of prescribing information: Elitek. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/103946s5083lbl.pdf (2009).
European Medicines Agency. Fasturtec (rasburicase). European Medicines Agency https://www.ema.europa.eu/en/medicines/human/EPAR/fasturtec (2023).
Allen, K. C. et al. Risk of anaphylaxis with repeated courses of rasburicase: a Research on Adverse Drug Events and Reports (RADAR) project. Drug Saf. 38, 183–187 (2015).
Richette, P., Brière, C., Hoenen-Clavert, V., Loeuille, D. & Bardin, T. Rasburicase for tophaceous gout not treatable with allopurinol: an exploratory study. J. Rheumatol. 34, 2093–2098 (2007).
Sherman, M. R., Saifer, M. G. & Perez-Ruiz, F. PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv. Drug Deliv. Rev. 60, 59–68 (2008).
Schlesinger, N., Yasothan, U. & Kirkpatrick, P. Pegloticase. Nat. Rev. Drug Discov. 10, 17–18 (2011).
Fels, E. & Sundy, J. S. Refractory gout: What is it and what to do about it? Curr. Opin. Rheumatol. 20, 198–202 (2008).
Doherty, M. et al. Gout: why is this curable disease so seldom cured? Ann. Rheum. Dis. 71, 1765–1770 (2012).
Schlesinger, N. & Lipsky, P. E. Pegloticase treatment of chronic refractory gout: update on efficacy and safety. Semin. Arthritis Rheum. 50, S31–S38 (2020).
Becker, M. A. et al. Long-term safety of pegloticase in chronic gout refractory to conventional treatment. Ann. Rheum. Dis. 72, 1469–1474 (2013).
Becker, M. A. et al. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. A twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 52, 916–923 (2005).
Cipolletta, E. et al. Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA 328, 440–450 (2022).
Hershfield, M. S. et al. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 16, R63 (2014).
Baraf, H. S., Yood, R. A., Ottery, F. D., Sundy, J. S. & Becker, M. A. Infusion-related reactions with pegloticase, a recombinant uricase for the treatment of chronic gout refractory to conventional therapy. J. Clin. Rheumatol. 20, 427–432 (2014).
Browning, L. A. & Kruse, J. A. Hemolysis and methemoglobinemia are secondary to rasburicase administration. Ann. Pharmacother. 39, 1932–1935 (2005).
Dean, L. & Kane M. in Medical Genetics Summaries (eds Pratt V. M. et al.) 489–498 (National Center for Biotechnology Information, 2020).
FDA. Highlights of prescribing information: Krystexxa. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125293s104lbl.pdf (2022).
Botson, J. K. et al. Pegloticase in combination with methotrexate in patients with uncontrolled gout: a multicenter, open-label study (MIRROR). J. Rheumatol. 48, 767–774 (2021).
Lamoreaux, B., Francis-Sedlak, M., Svensson, K. & Holt, R. Immunomodulation co-therapy with pegloticase: database trends 2014–2019 [abstract OP0173]. Ann. Rheum. Dis. 79, 108 (2020).
Khanna, P. P. et al. Reducing immunogenicity of pegloticase with concomitant use of mycophenolate mofetil in patients with refractory gout: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 73, 1523–1532 (2021).
Rainey, H., Baraf, H. S. B., Yeo, A. & Lipsky, P. E. Companion immunosuppression with azathioprine increases the frequency of persistent responsiveness to pegloticase in patients with chronic refractory gout [abstract THU0410]. Ann. Rheum. Dis. 79, 442–443 (2020).
Keenan, R. T. et al. The effect of immunomodulators on the efficacy and tolerability of pegloticase: a systematic review. Semin. Arthritis Rheum. 51, 347–352 (2021).
Botson, J. K. et al. A randomized, double-blind, placebo-controlled multicenter efficacy and safety study of methotrexate to increase response rates in patients with uncontrolled gout receiving pegloticase: 12-month findings. ACR Open Rheumatol. 5, 407–418 (2023).
Baraf, H. S. et al. Tophus burden reduction with pegloticase: results from phase 3 randomized trials and open-label extension in patients with chronic gout refractory to conventional therapy. Arthritis Res. Ther. 15, R137 (2013).
Botson, J. K. et al. A randomized, placebo-controlled study of methotrexate to increase response rates in patients with uncontrolled gout receiving pegloticase: primary efficacy and safety findings. Arthritis Rheumatol. 75, 293–304 (2023).
Dalbeth, N. et al. Reduction in monosodium urate crystal deposit volume during the MIRROR RCT trial in patients treated with pegloticase plus methotrexate co-therapy: a serial dual-energy computed tomography (DECT) analysis [abstract]. Arthritis Rheumatol. 74, 1807 (2022).
Strand, V., Khanna, D., Singh, J. A., Forsythe, A. & Edwards, N. L. Improved health-related quality of life and physical function in patients with refractory chronic gout following treatment with pegloticase: evidence from phase III randomized controlled trials. J. Rheumatol. 39, 1450–1457 (2012).
Sundström, J. et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 45, 28–33 (2005).
Cannon, P. J., Stason, W. B., Demartini, F. E., Sommers, S. C. & Laragh, J. H. Hyperuricemia in primary and renal hypertension. N. Engl. J. Med. 275, 457–464 (1966).
Kuwabara, M. et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: five-year Japanese cohort study. Hypertension 69, 1036–1044 (2017).
Soletsky, B. & Feig, D. I. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 60, 1148–1156 (2012).
Feig, D. I., Soletsky, B. & Johnson, R. J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300, 924–932 (2008).
Gaffo, A. L. et al. Effect of serum urate lowering with allopurinol on blood pressure in young adults: a randomized, controlled, crossover trial. Arthritis Rheumatol. 73, 1514–1522 (2021).
McMullan, C. J., Borgi, L., Fisher, N., Curhan, G. & Forman, J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: a randomized controlled trial. Clin. J. Am. Soc. Nephrol. 12, 807–816 (2017).
Doria, A. et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 382, 2493–2503 (2020).
Badve, S. V. et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 382, 2504–2513 (2020).
Mackenzie, I. S. et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 400, 1195–1205 (2022).
Doherty et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 392, 1403–1412 (2018).
White, W. B. et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 378, 1200–1210 (2018).
Mackenzie, I. S. et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 396, 1745–1757 (2020).
Johnson, R. J., Choi, H. K., Yeo, A. E. & Lipsky, P. E. Pegloticase treatment significantly decreases blood pressure in patients with chronic gout. Hypertension 74, 95–101 (2019).
Lanaspa, M. et al. Uric acid and hypertension. Hypertens. Res. 43, 832–834 (2020).
Bardin, T. et al. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int. 99, 218–226 (2021).
Bardin, T., Letavernier, E. & Correas, J.-M. The gouty kidney: a reappraisal. Gout Urate Cryst. Depos. Dis. 1, 25–36 (2023).
Stamp, L. K. et al. Management of gout in chronic kidney disease: a G-CAN Consensus Statement on the research priorities. Nat. Rev. Rheumatol. 17, 633–641 (2021).
Kuo, C. F. et al. Gout and risk of non-alcoholic fatty liver disease. Scand. J. Rheumatol. 39, 466–471 (2010).
Fernández Rodríguez, C. M. et al. Higher levels of serum uric acid influences hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD). Rev. Esp. Enferm. Dig. 111, 264–269 (2019).
Sandra, S. et al. Hyperuricemia as an independent risk factor for non-alcoholic fatty liver disease (NAFLD) progression evaluated using controlled attenuation parameter-transient elastography: Lesson learnt from a tertiary referral center. Diabetes Metab. Syndr. 13, 424–428 (2019).
Schlesinger, N., Rustgi, V. K., Yeo, A. E. & Lipsky, P. E. Improvement in hepatic fibrosis estimated by Fibrosis-4 index in pegloticase treated chronic refractory gout patients. Clin. Exp. Rheumatol. 41, 60–66 (2023).
Szabo, G. & Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 12, 387–400 (2015).
Sands, E. et al. Tolerogenic nanoparticles mitigate the formation of anti-drug antibodies against pegylated uricase in patients with hyperuricemia. Nat. Commun. 13, 272 (2022).
Azeem, R. et al. Initial phase 2 clinical data of SEL-212 in symptomatic gout patients: monthly dosing of a pegylated uricase (pegadricase) with Svp-rapamycin enables sustained reduction of acute gout flares [abstract]. Arthritis Rheumatol. 70, 1294 (2018).
Baraf, H. S. B. et al. The COMPARE head-to-head, randomised-controlled trial of SEL-212 (pegadricase plus rapamycin-containing nanoparticle, ImmTOR™) versus pegloticase for refractory gout. Rheumatology https://doi.org/10.1093/rheumatology/kead333 (2023).
Baraf, H. S. B. et al. Safety & efficacy of SEL-212 in patients with gout refractory to coventional treatment: outcomes from two randomized, double blind, placebo-controlled, multicenter phase III studies [abstract LB0002]. Ann. Rheum. Dis. 82, 200–201 (2023).
Pierzynowska, K. et al. Oral treatment with an engineered uricase, ALLN-346, reduces hyperuricemia, and uricosuria in urate oxidase-deficient mice. Front. Med. 7, 569215 (2020).
Terkeltaub, R. et al.Safety and efficacy of ALLN-346 oral enzyme therapy in patients with hyperuricemia and chronic kidney disease (CKD): results of the phase 2A study 201 [abstract POS1157]. Ann. Rheum. Dis. 81, 907 (2022).
Hanania, U. et al. Establishment of a tobacco BY2 cell line devoid of plant-specific xylose and fucose as a platform for the production of biotherapeutic proteins. Plant. Biotechnol. J. 15, 1120–1129 (2017).
Atsmon, J. et al. Preclinical and first-in-human evaluation of PRX-105, a PEGylated, plant-derived, recombinant human acetylcholinesterase-R. Toxicol. Appl. Pharmacol. 287, 202–209 (2015).
Schlesinger, N., Etzel, C., Greenberg, J., Kremer, J. & Harrold, L. Gout flare prophylaxis evaluated according to the 2012 American College of Rheumatology (ACR) guidelines: analysis from the CORRONA Gout Registry. J. Rheumatol. 43, 924–930 (2016).
Schlesinger, N. Treatment of chronic gouty arthritis: it’s not just about urate-lowering therapy. Semin. Arthritis Rheum. 42, 155–165 (2012).
FDA. Highlights of prescribing information: Colcrys. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022353lbl.pdf (2009).
European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of gout. European Medicines Agency https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-gout-first-version_en.pdf (2019).
Schlesinger, N. et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol therapy: results of a double-blind, randomised study. Ann. Rheum. Dis. 70, 1264–1271 (2011).
Mitha, E. et al. Rilonacept for gout flare prevention during initiation of uric acid-lowering therapy: results from the PRESURGE-2 international, phase 3, randomized, placebo-controlled trial. Rheumatology 52, 1285–1292 (2013).
Keenan, R. T., Baraf, H. S. B. & LaMoreaux, B. Use of pre-infusion serum uric acid levels as a biomarker for infusion reaction risk in patients on pegloticase. Rheumatol. Ther. 6, 299–304 (2019).
Schlesinger, I. & Schlesinger, N. Uric acid in Parkinson’s disease. Mov. Disord. 23, 1653–1657 (2008).
Li, Z., Hoshino, Y., Tran, L. & Gaucher, E. A. Phylogenetic articulation of uric acid evolution in mammals and how it informs a therapeutic uricase. Mol. Biol. Evol. 39, msab312 (2022).
Wu, X. W., Muzny, D. M., Lee, C. C. & Caskey, C. T. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J. Mol. Evol. 34, 78–84 (1992).
Eaton, S. B. & Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 312, 283–289 (1985).
Powles, J. et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3, e003733 (2013).
Watanabe, S. et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 40, 355–360 (2002).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
N.S. declares that she has received a grant from Olatec and has been on advisory boards and acted as a consultant for ANI, Horizon Pharma, Novartis, Protalix and SOBI. F.P.-R. declares that he is an adviser for Anlylam, Arthriti and Protalix; a speaker for Menarini Central America; a member of Data and Safety Monitoring Board (DMSB) for Selecta/SOBI; does editorial work for Springer Healthcare, Wolters-Kluwer, Exploration Publications and the Spanish Foundation for Rheumatology; and has received grants from the Cruces Rheumatology Association. F.L. declares that he has been on advisory boards for Horizon Pharma, Novartis France and Olatec; is a member of DMSB for Olatec; does editorial work for Elsevier; is a CME speaker for Mayoly-Spindler, Menarini Central America and Novartis; and has received unrestricted educational grants from Horizon Pharma, Mayoly-Spindler, Novartis, Olatec and SOBI for the European Crystal Network workshops.
Peer review
Peer review information
Nature Reviews Rheumatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schlesinger, N., Pérez-Ruiz, F. & Lioté, F. Mechanisms and rationale for uricase use in patients with gout. Nat Rev Rheumatol 19, 640–649 (2023). https://doi.org/10.1038/s41584-023-01006-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01006-3
This article is cited by
-
Dos and Don’ts in der rheumatologischen Primärversorgung
rheuma plus (2024)