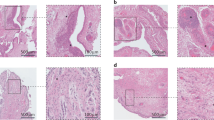
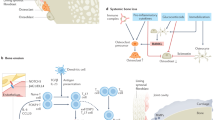
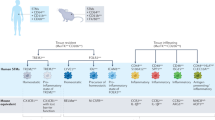
Abstract
Current treatments for rheumatoid arthritis (RA) do not work well for a large proportion of patients, or at all in some individuals, and cannot cure or prevent this disease. One major obstacle to developing better drugs is a lack of complete understanding of how inflammatory joint disease arises and progresses. Emerging evidence indicates an important role for the tissue microenvironment in the pathogenesis of RA. Each tissue is made up of cells surrounded and supported by a unique extracellular matrix (ECM). These complex molecular networks define tissue architecture and provide environmental signals that programme site-specific cell behaviour. In the synovium, a main site of disease activity in RA, positional and disease stage-specific cellular diversity exist. Improved understanding of the architecture of the synovium from gross anatomy to the single-cell level, in parallel with evidence demonstrating how the synovial ECM is vital for synovial homeostasis and how dysregulated signals from the ECM promote chronic inflammation and tissue destruction in the RA joint, has opened up new ways of thinking about the pathogenesis of RA. These new ideas provide novel therapeutic approaches for patients with difficult-to-treat disease and could also be used in disease prevention.
Key points
-
All tissues are made up of cells surrounded by an extracellular matrix (ECM), an intricate 3D molecular network that is an important determinant of tissue architecture and cell behaviour.
-
The synovium is a complex anatomical tissue comprising many cell (sub)populations that are located in distinct sub-synovial niches, each of which are specialized to perform unique roles in synovial homeostasis.
-
In rheumatoid arthritis (RA), infiltrating immune cells join tissue-resident cells, leading to qualitative changes in cell phenotype that promote inflammation and tissue destruction, and suppress the resolution of inflammation.
-
The ECM has an important role in dictating the organization of synovial cell networks and in programming synovial cell specialization.
-
Changes in the synovial microenvironment start to occur early in the development of RA, and these aberrant extracellular cues shape pathogenic cell behaviour during the onset and progression of disease.
-
Analysing localized changes in the synovium can improve disease classification and patient stratification, and targeting the ECM holds promise for the development of new strategies to treat and prevent RA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
12 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41584-021-00602-5
References
Human Cell Atlas https://www.humancellatlas.org (2020).
Amit, I., Winter, D. R. & Jung, S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat. Immunol. 17, 18–25 (2016).
Chang, H. Y. et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. USA 99, 12877–12882 (2002).
Naba, A. et al. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 49, 10–24 (2016).
Naba, A., Ding, H., Whittaker, C. A. & Hynes, R. O. Matrisome Project http://www.matrisomeproject.mit.edu (2020).
Smith, M. D. The normal synovium. Open Rheumatol. J. 5, 100–106 (2011).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Pitzalis, C., Kelly, S. & Humby, F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 25, 334–344 (2013).
Nerviani, A. & Pitzalis, C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J. Leukoc. Biol. 104, 333–341 (2018).
Dennis, G. Jr. et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 16, R90 (2014).
Lewis, M. J. et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 28, 2455–2470 (2019).
O’Sullivan, F. X., Fassbender, H. G., Gay, S. & Koopman, W. J. Etiopathogenesis of the rheumatoid arthritis-like disease in MRL/l mice. I. The histomorphologic basis of joint destruction. Arthritis Rheum. 28, 529–536 (1985).
Geiler, T., Kriegsmann, J., Keyszer, G. M., Gay, R. E. & Gay, S. A new model for rheumatoid arthritis generated by engraftment of rheumatoid synovial tissue and normal human cartilage into SCID mice. Arthritis Rheum. 37, 1664–1671 (1994).
Muller-Ladner, U. et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149, 1607–1615 (1996).
Kurowska-Stolarska, M. et al. Inhibitor of DNA binding/differentiation 2 induced by hypoxia promotes synovial fibroblast-dependent osteoclastogenesis. Arthritis Rheum. 60, 3663–3675 (2009).
Jungel, A. et al. Effect of the oral application of a highly selective MMP-13 inhibitor in three different animal models of rheumatoid arthritis. Ann. Rheum. Dis. 69, 898–902 (2010).
Pap, T. et al. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 43, 1226–1232 (2000).
Seibl, R. et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 162, 1221–1227 (2003).
Firestein, G. S. et al. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 149, 2143–2151 (1996).
Seemayer, C. A. et al. p53 in rheumatoid arthritis synovial fibroblasts at sites of invasion. Ann. Rheum. Dis. 62, 1139–1144 (2003).
Franz, J. K. et al. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 43, 599–607 (2000).
Pap, T. et al. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2, 59–64 (2000).
Neidhart, M. et al. Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum. 43, 2634–2647 (2000).
Karouzakis, E., Gay, R. E., Gay, S. & Neidhart, M. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat. Rev. Rheumatol. 5, 266–272 (2009).
Mendez-Huergo, S. P. et al. Clinical relevance of galectin-1 and galectin-3 in rheumatoid arthritis patients: differential regulation and correlation with disease activity. Front. Immunol. 9, 3057 (2018).
Ohshima, S. et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 48, 2788–2795 (2003).
Neidhart, M. et al. Galectin-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric matrix protein. Ann. Rheum. Dis. 64, 419–424 (2005).
Filer, A. et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 60, 1604–1614 (2009).
Arad, U. et al. Galectin-3 is a sensor-regulator of Toll-like receptor pathways in synovial fibroblasts. Cytokine 73, 30–35 (2015).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 9, 791 (2018).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Littman, D. R. & Rudensky, A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Culemann, S. et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572, 670–675 (2019).
Alivernini, S. et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 26, 1295–1306 (2020).
Kuo, D. et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl Med. 11, eaau8587 (2019).
Wei, K. et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
Filer, A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr. Opin. Pharmacol. 13, 413–419 (2013).
Sherlock, J. P., Filer, A. D., Isaacs, J. D. & Buckley, C. D. What can rheumatologists learn from translational cancer therapy? Arthritis Res. Ther. 15, 114 (2013).
Klein, K. et al. Evaluating the bromodomain protein BRD1 as a therapeutic target in rheumatoid arthritis. Sci. Rep. 8, 11125 (2018).
Neidhart, M., Karouzakis, E., Jungel, A., Gay, R. E. & Gay, S. Inhibition of spermidine/spermine N1-acetyltransferase activity: a new therapeutic concept in rheumatoid arthritis. Arthritis Rheumatol. 66, 1723–1733 (2014).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Tamer, T. M. Hyaluronan and synovial joint: function, distribution and healing. Interdiscip. Toxicol. 6, 111–125 (2013).
Jay, G. D. & Waller, K. A. The biology of lubricin: near frictionless joint motion. Matrix Biol. 39, 17–24 (2014).
Gay, S., Gay, R. E. & Miller, E. F. The collagens of the joint. Arthritis Rheum. 23, 937–941 (1980).
Ouboussad, L., Burska, A. N., Melville, A. & Buch, M. H. Synovial tissue heterogeneity in rheumatoid arthritis and changes with biologic and targeted synthetic therapies to inform stratified therapy. Front. Med. 6, 45 (2019).
Miller, A. E., Hu, P. & Barker, T. H. Feeling things out: bidirectional signaling of the cell–ECM interface, implications in the mechanobiology of cell spreading, migration, proliferation, and differentiation. Adv. Healthc. Mater. 9, e1901445 (2020).
Qu, F., Guilak, F. & Mauck, R. L. Cell migration: implications for repair and regeneration in joint disease. Nat. Rev. Rheumatol. 15, 167–179 (2019).
Jain, N., Moeller, J. & Vogel, V. Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu. Rev. Biomed. Eng. 21, 267–297 (2019).
Piersma, B., Hayward, M. K. & Weaver, V. M. Fibrosis and cancer: a strained relationship. Biochim. Biophys. Acta Rev. Cancer 1873, 188356 (2020).
Northcott, J. M., Dean, I. S., Mouw, J. K. & Weaver, V. M. Feeling stress: the mechanics of cancer progression and aggression. Front. Cell Dev. Biol. 6, 17 (2018).
Shelef, M. A., Bennin, D. A., Mosher, D. F. & Huttenlocher, A. Citrullination of fibronectin modulates synovial fibroblast behavior. Arthritis Res. Ther. 14, R240 (2012).
van Dinther-Janssen, A. C., Pals, S. T., Scheper, R. J. & Meijer, C. J. Role of the CS1 adhesion motif of fibronectin in T cell adhesion to synovial membrane and peripheral lymph node endothelium. Ann. Rheum. Dis. 52, 672–676 (1993).
Simon, M. M., Kramer, M. D., Prester, M. & Gay, S. Mouse T-cell associated serine proteinase 1 degrades collagen type IV: a structural basis for the migration of lymphocytes through vascular basement membranes. Immunology 73, 117–119 (1991).
Mueller, S. C. & Chen, W. T. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J. Cell Sci. 99, 213–225 (1991).
Lubberts, E. & van den Berg, W. B. Cytokines in the pathogenesis of rheumatoid arthritis and collagen-induced arthritis. Adv. Exp. Med. Biol. 520, 194–202 (2003).
Middleton, J., Patterson, A. M., Gardner, L., Schmutz, C. & Ashton, B. A. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood 100, 3853–3860 (2002).
Sadir, R., Imberty, A., Baleux, F. & Lortat-Jacob, H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J. Biol. Chem. 279, 43854–43860 (2004).
Johnson, Z. et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J. Immunol. 173, 5776–5785 (2004).
Kehoe, O. et al. Syndecan-3 is selectively pro-inflammatory in the joint and contributes to antigen-induced arthritis in mice. Arthritis Res. Ther. 16, R148 (2014).
Patterson, A. M. et al. Induction of a CXCL8 binding site on endothelial syndecan-3 in rheumatoid synovium. Arthritis Rheum. 52, 2331–2342 (2005).
Martino, M. M. et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885–888 (2014).
LeBaron, R. G., Hook, A., Esko, J. D., Gay, S. & Hook, M. Binding of heparan sulfate to type V collagen. A mechanism of cell-substrate adhesion. J. Biol. Chem. 264, 7950–7956 (1989).
Forsten-Williams, K., Chu, C. L., Fannon, M., Buczek-Thomas, J. A. & Nugent, M. A. Control of growth factor networks by heparan sulfate proteoglycans. Ann. Biomed. Eng. 36, 2134–2148 (2008).
Mythreye, K. & Blobe, G. C. Proteoglycan signaling co-receptors: roles in cell adhesion, migration and invasion. Cell Signal. 21, 1548–1558 (2009).
Pap, T. & Bertrand, J. Syndecans in cartilage breakdown and synovial inflammation. Nat. Rev. Rheumatol. 9, 43–55 (2013).
Shao, X. et al. FGF2 cooperates with IL-17 to promote autoimmune inflammation. Sci. Rep. 7, 7024 (2017).
Elfenbein, A. & Simons, M. Syndecan-4 signaling at a glance. J. Cell Sci. 126, 3799–3804 (2013).
Bazzoni, F. & Beutler, B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 334, 1717–1725 (1996).
Rico, M. C. et al. Thrombospondin-1 and transforming growth factor beta are pro-inflammatory molecules in rheumatoid arthritis. Transl Res. 152, 95–98 (2008).
Suzuki, T. et al. Upregulation of thrombospondin 1 expression in synovial tissues and plasma of rheumatoid arthritis: role of transforming growth factor-beta1 toward fibroblast-like synovial cells. J. Rheumatol. 42, 943–947 (2015).
Resovi, A., Pinessi, D., Chiorino, G. & Taraboletti, G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 37, 83–91 (2014).
Nefla, M., Holzinger, D., Berenbaum, F. & Jacques, C. The danger from within: alarmins in arthritis. Nat. Rev. Rheumatol. 12, 669–683 (2016).
Frevert, C. W., Felgenhauer, J., Wygrecka, M., Nastase, M. V. & Schaefer, L. Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J. Histochem. Cytochem. 66, 213–227 (2018).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Goh, F. G., Piccinini, A. M., Krausgruber, T., Udalova, I. A. & Midwood, K. S. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J. Immunol. 184, 2655–2662 (2010).
Marzeda, A. M. & Midwood, K. S. Internal affairs: tenascin-C as a clinically relevant, endogenous driver of innate immunity. J. Histochem. Cytochem. 66, 289–304 (2018).
Zuliani-Alvarez, L. et al. Mapping tenascin-C interaction with Toll-like receptor 4 reveals a new subset of endogenous inflammatory triggers. Nat. Commun. 8, 1595 (2017).
Li, Y. et al. Identification of potential genetic causal variants for rheumatoid arthritis by whole-exome sequencing. Oncotarget 8, 111119–111129 (2017).
Xiong, Y. et al. Bioinformatics analysis and identification of genes and molecular pathways involved in synovial inflammation in rheumatoid arthritis. Med. Sci. Monit. 25, 2246–2256 (2019).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Karouzakis, E., Neidhart, M., Gay, R. E. & Gay, S. Molecular and cellular basis of rheumatoid joint destruction. Immunol. Lett. 106, 8–13 (2006).
Garnero, P., Rousseau, J. C. & Delmas, P. D. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 43, 953–968 (2000).
Karsdal, M. A. et al. Biochemical markers of ongoing joint damage in rheumatoid arthritis — current and future applications, limitations and opportunities. Arthritis Res. Ther. 13, 215 (2011).
Aschenberg, S. et al. Catabolic and anabolic periarticular bone changes in patients with rheumatoid arthritis: a computed tomography study on the role of age, disease duration and bone markers. Arthritis Res. Ther. 15, R62 (2013).
Chapurlat, R. D. & Confavreux, C. B. Novel biological markers of bone: from bone metabolism to bone physiology. Rheumatology 55, 1714–1725 (2016).
Saxne, T. & Heinegard, D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br. J. Rheumatol. 31, 583–591 (1992).
Christensen, A. F. et al. Differential association of the N-propeptide of collagen IIA (PIIANP) and collagen II C-telopeptide (CTX-II) with synovitis and erosions in early and longstanding rheumatoid arthritis. Clin. Exp. Rheumatol. 27, 307–314 (2009).
Bay-Jensen, A. C. et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM — increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin. Biochem. 44, 423–429 (2011).
Barascuk, N. et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin. Biochem. 43, 899–904 (2010).
Bay-Jensen, A. C. et al. Circulating protein fragments of cartilage and connective tissue degradation are diagnostic and prognostic markers of rheumatoid arthritis and ankylosing spondylitis. PLoS ONE 8, e54504 (2013).
Gudmann, N. S. et al. Increased remodelling of interstitial collagens and basement membrane is suppressed by treatment in patients with rheumatoid arthritis: serological evaluation of a one-year prospective study of 149 Japanese patients. Clin. Exp. Rheumatol. 36, 462–470 (2018).
Leeming, D. et al. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M). Biomarkers 16, 616–628 (2011).
Ma, J. D. et al. Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediators Inflamm. 2014, 179284 (2014).
Sun, S. et al. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet. Disord. 15, 93 (2014).
Bay-Jensen, A. C. et al. Serological biomarkers of joint tissue turnover predict tocilizumab response at baseline. J. Clin. Rheumatol. 20, 332–335 (2014).
Bay-Jensen, A. C. et al. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin. Arthritis Rheum. 43, 470–478 (2014).
Gudmann, N. S. et al. Type IV collagen metabolism is associated with disease activity, radiographic progression and response to tocilizumab in rheumatoid arthritis. Clin. Exp. Rheumatol. 36, 829–835 (2018).
Juhl, P. et al. IL-6 receptor inhibition modulates type III collagen and C-reactive protein degradation in rheumatoid arthritis patients with an inadequate response to anti-tumour necrosis factor therapy: analysis of connective tissue turnover in the tocilizumab RADIATE study. Clin. Exp. Rheumatol. 36, 568–574 (2018).
Kjelgaard-Petersen, C. F. et al. Translational biomarkers and ex vivo models of joint tissues as a tool for drug development in rheumatoid arthritis. Arthritis Rheumatol. 70, 1419–1428 (2018).
Majkowska, I., Shitomi, Y., Ito, N., Gray, N. S. & Itoh, Y. Discoidin domain receptor 2 mediates collagen-induced activation of membrane-type 1 matrix metalloproteinase in human fibroblasts. J. Biol. Chem. 292, 6633–6643 (2017).
Nagy, N. et al. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 78-79, 292–313 (2019).
Scheibner, K. A. et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 (2006).
Hasegawa, M. et al. Thrombin-cleaved osteopontin in synovial fluid of subjects with rheumatoid arthritis. J. Rheumatol. 36, 240–245 (2009).
Kazanecki, C. C., Uzwiak, D. J. & Denhardt, D. T. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J. Cell Biochem. 102, 912–924 (2007).
Weber, G. F. et al. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 72, 752–761 (2002).
Luukkonen, J. et al. Increased amount of phosphorylated proinflammatory osteopontin in rheumatoid arthritis synovia is associated to decreased tartrate-resistant acid phosphatase 5B/5A ratio. PLoS ONE 12, e0182904 (2017).
Wegner, N. et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev. 233, 34–54 (2010).
Foster, M. H. Basement membranes and autoimmune diseases. Matrix Biol. 57–58, 149–168 (2017).
Steen, J. et al. Recognition of amino acid motifs, rather than specific proteins, by human plasma cell-derived monoclonal antibodies to posttranslationally modified proteins in rheumatoid arthritis. Arthritis Rheumatol. 71, 196–209 (2019).
Haag, S. et al. Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type II collagen. Arthritis Rheumatol. 66, 1440–1449 (2014).
Burkhardt, H. et al. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 46, 2339–2348 (2002).
Holmdahl, R., Jansson, L., Larsson, A. & Jonsson, R. Arthritis in DBA/1 mice induced with passively transferred type II collagen immune serum. Immunohistopathology and serum levels of anti-type II collagen auto-antibodies. Scand. J. Immunol. 31, 147–157 (1990).
Raats, J. M., Wijnen, E. M., Pruijn, G. J., van den Hoogen, F. H. & van Venrooij, W. J. Recombinant human monoclonal autoantibodies specific for citrulline-containing peptides from phage display libraries derived from patients with rheumatoid arthritis. J. Rheumatol. 30, 1696–1711 (2003).
Boman, A. et al. Antibodies against citrullinated peptides are associated with clinical and radiological outcomes in patients with early rheumatoid arthritis: a prospective longitudinal inception cohort study. RMD Open 5, e000946 (2019).
Schwenzer, A. et al. Identification of an immunodominant peptide from citrullinated tenascin-C as a major target for autoantibodies in rheumatoid arthritis. Ann. Rheum. Dis. 75, 1876–1883 (2016).
Schwenzer, A. et al. Association of distinct fine specificities of anti-citrullinated peptide antibodies with elevated immune responses to Prevotella intermedia in a subgroup of patients with rheumatoid arthritis and periodontitis. Arthritis Rheumatol. 69, 2303–2313 (2017).
Rims, C. et al. Citrullinated aggrecan epitopes as targets of autoreactive CD4+ T cells in patients with rheumatoid arthritis. Arthritis Rheumatol. 71, 518–528 (2019).
Stefanelli, V. L. et al. Citrullination of fibronectin alters integrin clustering and focal adhesion stability promoting stromal cell invasion. Matrix Biol. 82, 86–104 (2019).
Lundberg, K. et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res. Ther. 7, R458–R467 (2005).
Vossenaar, E. R. et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 48, 2489–2500 (2003).
Ho, P. P. et al. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J. Immunol. 184, 379–390 (2010).
Fan, L. et al. Citrullinated fibronectin inhibits apoptosis and promotes the secretion of pro-inflammatory cytokines in fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res. Ther. 14, R266 (2012).
Sanchez-Pernaute, O. et al. Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Ann. Rheum. Dis. 72, 1400–1406 (2013).
Sokolove, J., Zhao, X., Chandra, P. E. & Robinson, W. H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 63, 53–62 (2011).
Sipila, K. et al. Citrullination of collagen II affects integrin-mediated cell adhesion in a receptor-specific manner. FASEB J. 28, 3758–3768 (2014).
Chang, X. et al. Citrullination of fibronectin in rheumatoid arthritis synovial tissue. Rheumatology 44, 1374–1382 (2005).
Yan, X., Yin, L., Wang, Y., Zhao, Y. & Chang, X. The low binding affinity of ADAMTS4 for citrullinated fibronectin may contribute to the destruction of joint cartilage in rheumatoid arthritis. Clin. Exp. Rheumatol. 31, 201–206 (2013).
Zoumi, A., Yeh, A. & Tromberg, B. J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl Acad. Sci. USA 99, 11014–11019 (2002).
Molleken, C. et al. MFAP4: a candidate biomarker for hepatic and pulmonary fibrosis? Sarcoidosis Vasc. Diffuse Lung Dis. 33, 41–50 (2016).
Christensen, A. F. et al. Site-specific absence of microfibrillar-associated protein 4 (MFAP4) from the internal elastic membrane of arterioles in the rheumatoid arthritis synovial membrane: an immunohistochemical study in patients with advanced rheumatoid arthritis versus osteoarthritis. APMIS 127, 588–593 (2019).
Hasegawa, M. et al. Expression of large tenascin-C splice variants in synovial fluid of patients with rheumatoid arthritis. J. Orthop. Res. 25, 563–568 (2007).
Page, T. H. et al. Raised circulating tenascin-C in rheumatoid arthritis. Arthritis Res. Ther. 14, R260 (2012).
Aungier, S. R. et al. Targeting early changes in the synovial microenvironment: a new class of immunomodulatory therapy? Ann. Rheum. Dis. 78, 186–191 (2019).
Asano, T. et al. α9β1 integrin acts as a critical intrinsic regulator of human rheumatoid arthritis. Rheumatology 53, 415–424 (2014).
Rupp, T. et al. Tenascin-C orchestrates glioblastoma angiogenesis by modulation of pro- and anti-angiogenic signaling. Cell Rep. 17, 2607–2619 (2016).
Kumar, A. et al. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 19, 1902–1916 (2017).
Orr, C. et al. Synovial tissue research: a state-of-the-art review. Nat. Rev. Rheumatol. 13, 463–475 (2017).
Muzard, J. et al. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS ONE 4, e5585 (2009).
Han, Z. & Lu, Z. R. Targeting fibronectin for cancer imaging and therapy. J. Mater. Chem. B 5, 639–654 (2017).
Baues, M. et al. Fibrosis imaging: current concepts and future directions. Adv. Drug Deliv. Rev. 121, 9–26 (2017).
Desogere, P., Montesi, S. B. & Caravan, P. Molecular probes for imaging fibrosis and fibrogenesis. Chemistry 25, 1128–1141 (2019).
Beziere, N. et al. Imaging fibrosis in inflammatory diseases: targeting the exposed extracellular matrix. Theranostics 9, 2868–2881 (2019).
Schultz, C. Targeting the extracellular matrix for delivery of bioactive molecules to sites of arthritis. Br. J. Pharmacol. 176, 26–37 (2019).
Schmid, A. S. & Neri, D. Advances in antibody engineering for rheumatic diseases. Nat. Rev. Rheumatol. 15, 197–207 (2019).
To, W. S. & Midwood, K. S. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 4, 21 (2011).
Bruijnen, S. T. G. et al. F8-IL10: A new potential antirheumatic drug evaluated by a PET-guided translational approach. Mol. Pharm. 16, 273–281 (2019).
Galeazzi, M. et al. A phase IB clinical trial with Dekavil (F8-IL10), an immunoregulatory ‘armed antibody’ for the treatment of rheumatoid arthritis, used in combination wiIh methotrexate. Isr. Med. Assoc. J. 16, 666 (2014).
Brennan, F. M. Interleukin 10 and arthritis. Rheumatology 38, 293–297 (1999).
Schwager, K. et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 11, R142 (2009).
Aalbers, C. et al. Intra-articular etanercept treatment in inflammatory arthritis: a randomized double-blind placebo-controlled proof of mechanism clinical trial validating TNF as a potential therapeutic target for local treatment. Joint Bone Spine 82, 338–344 (2015).
Wallis, W. J., Simkin, P. A. & Nelp, W. B. Protein traffic in human synovial effusions. Arthritis Rheum. 30, 57–63 (1987).
Katsumata, K. et al. Conferring extracellular matrix affinity enhances local therapeutic efficacy of anti-TNF-alpha antibody in a murine model of rheumatoid arthritis. Arthritis Res. Ther. 21, 298 (2019).
Katsumata, K. et al. Targeting inflammatory sites through collagen affinity enhances the therapeutic efficacy of anti-inflammatory antibodies. Sci. Adv. 5, eaay1971 (2019).
Lee, C. J. et al. Development of an inflammatory tissue-selective chimeric TNF receptor. Cytokine 113, 340–346 (2019).
Itoh, Y. Metalloproteinases in rheumatoid arthritis: potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Transl Sci. 148, 327–338 (2017).
Malemud, C. J. Matrix metalloproteinases and synovial joint pathology. Prog. Mol. Biol. Transl Sci. 148, 305–325 (2017).
Gossage, D. L. et al. Phase 1b study of the safety, pharmacokinetics, and disease-related outcomes of the matrix metalloproteinase-9 inhibitor andecaliximab in patients with rheumatoid arthritis. Clin. Ther. 40, 156–165 (2018).
Kaneko, K. et al. Selective inhibition of membrane type 1 matrix metalloproteinase abrogates progression of experimental inflammatory arthritis: Synergy with tumor necrosis factor blockade. Arthritis Rheumatol. 68, 521–531 (2016).
Falsone, A. et al. Designing CXCL8-based decoy proteins with strong anti-inflammatory activity in vivo. Biosci. Rep. 33, e00068 (2013).
McNaughton, E. F. et al. Novel anti-inflammatory peptides based on chemokine-glycosaminoglycan interactions reduce leukocyte migration and disease severity in a model of rheumatoid arthritis. J. Immunol. 200, 3201–3217 (2018).
Eustace, A. D. et al. Soluble syndecan-3 binds chemokines, reduces leukocyte migration in vitro and ameliorates disease severity in models of rheumatoid arthritis. Arthritis Res. Ther. 21, 172 (2019).
Take, Y. et al. Specifically modified osteopontin in rheumatoid arthritis fibroblast-like synoviocytes supports interaction with B cells and enhances production of interleukin-6. Arthritis Rheum. 60, 3591–3601 (2009).
Mehta, B. B. et al. Blocking osteopontin-fibronectin interactions reduce extracellular fibronectin deployment and arthritic immunopathology. Int. Immunopharmacol. 55, 297–305 (2018).
Ammitzboll, C. G. et al. M-ficolin levels reflect disease activity and predict remission in early rheumatoid arthritis. Arthritis Rheum. 65, 3045–3050 (2013).
Raza, K. et al. Detection of antibodies to citrullinated tenascin-C in patients with early synovitis is associated with the development of rheumatoid arthritis. RMD Open 2, e000318 (2016).
Cutolo, M., Soldano, S. & Paolino, S. Potential roles for tenascin in (very) early diagnosis and treatment of rheumatoid arthritis. Ann. Rheum. Dis. 79, e42 (2020).
Filipe, E. C., Chitty, J. L. & Cox, T. R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 99, 58–76 (2018).
Taha, I. N. & Naba, A. Exploring the extracellular matrix in health and disease using proteomics. Essays Biochem. 63, 417–432 (2019).
van den Brink, S. C. et al. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat. Methods 14, 935–936 (2017).
van Velthoven, C. T. J., de Morree, A., Egner, I. M., Brett, J. O. & Rando, T. A. Transcriptional profiling of quiescent muscle stem cells in vivo. Cell Rep. 21, 1994–2004 (2017).
Medaglia, C. et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science 358, 1622–1626 (2017).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Rocha, B., Cillero-Pastor, B., Blanco, F. J. & Ruiz-Romero, C. MALDI mass spectrometry imaging in rheumatic diseases. Biochim. Biophys. Acta Proteins Proteom. 1865, 784–794 (2017).
Chakraborty, T. et al. Light-sheet microscopy of cleared tissues with isotropic, subcellular resolution. Nat. Methods 16, 1109–1113 (2019).
Lavin, Y. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Klein, K. et al. The epigenetic architecture at gene promoters determines cell type-specific LPS tolerance. J. Autoimmun. 83, 122–133 (2017).
Ospelt, C. et al. Overexpression of Toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 58, 3684–3692 (2008).
Crowley, T. et al. Priming in response to pro-inflammatory cytokines is a feature of adult synovial but not dermal fibroblasts. Arthritis Res. Ther. 19, 35 (2017).
Frank-Bertoncelj, M. et al. Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nat. Commun. 8, 14852 (2017).
Rinn, J. L., Bondre, C., Gladstone, H. B., Brown, P. O. & Chang, H. Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2, e119 (2006).
Higuchi, Y. et al. Gastrointestinal fibroblasts have specialized, diverse transcriptional phenotypes: a comprehensive gene expression analysis of human fibroblasts. PLoS ONE 10, e0129241 (2015).
Hsueh, M. F., Onnerfjord, P., Bolognesi, M. P., Easley, M. E. & Kraus, V. B. Analysis of “old” proteins unmasks dynamic gradient of cartilage turnover in human limbs. Sci. Adv. 5, eaax3203 (2019).
Quinn, T. M., Hauselmann, H. J., Shintani, N. & Hunziker, E. B. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthritis Cartilage 21, 1904–1912 (2013).
Treppo, S. et al. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J. Orthop. Res. 18, 739–748 (2000).
Ai, R. et al. Joint-specific DNA methylation and transcriptome signatures in rheumatoid arthritis identify distinct pathogenic processes. Nat. Commun. 7, 11849 (2016).
den Hollander, W. et al. Knee and hip articular cartilage have distinct epigenomic landscapes: implications for future cartilage regeneration approaches. Ann. Rheum. Dis. 73, 2208–2212 (2014).
Felsenthal, N. & Zelzer, E. Mechanical regulation of musculoskeletal system development. Development 144, 4271–4283 (2017).
Schroder, A. et al. Impact of mechanical load on the expression profile of synovial fibroblasts from patients with and without osteoarthritis. Int. J. Mol. Sci. 20, 585 (2019).
Shimomura, K. et al. Cyclic compressive loading on 3D tissue of human synovial fibroblasts upregulates prostaglandin E2 via COX-2 production without IL-1beta and TNF-alpha. Bone Joint Res. 3, 280–288 (2014).
Gentili, C. & Cancedda, R. Cartilage and bone extracellular matrix. Curr. Pharm. Des. 15, 1334–1348 (2009).
Riley, G. Tendinopathy — from basic science to treatment. Nat. Clin. Pract. Rheumatol. 4, 82–89 (2008).
Acknowledgements
The work of the C.D.B. is supported by the National Institute for Health Research through the Birmingham Biomedical Research Centre and Wellcome Trust Clinical Research Facility at University Hospitals Birmingham NHS Foundation Trust. Funding was also provided by the Versus Arthritis RACE Rheumatoid Arthritis Pathogenesis Centre of Excellence (grant 20298), a Versus Arthritis Programme grant to C.D.B. (grant 19791), a Versus Arthritis Senior Fellowship to K.S.M. (grant 20003) and from the Swiss National Science Foundation to C.O. (project 320030_176061).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
C.D.B. declares that he is a founder of MesTag Limited and has received funding from MesTag. C.O. declares that she has received consultancy fees from Gilead Sciences and funding from Novartis. K.S.M. declares that she is the founder and director of Nascient Limited and has received research funding from Nascient. S.G. declares no competing interests.
Additional information
Disclaimer
The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, the authors’ funding bodies or the Department of Health.
Peer review information
Nature Reviews Rheumatology thanks L. Donlin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buckley, C.D., Ospelt, C., Gay, S. et al. Location, location, location: how the tissue microenvironment affects inflammation in RA. Nat Rev Rheumatol 17, 195–212 (2021). https://doi.org/10.1038/s41584-020-00570-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-00570-2
This article is cited by
-
Prodrug-based nanomedicines for rheumatoid arthritis
Discover Nano (2024)
-
Axl and MerTK regulate synovial inflammation and are modulated by IL-6 inhibition in rheumatoid arthritis
Nature Communications (2024)
-
Synovial Tissue Insights into Heterogeneity of Rheumatoid Arthritis
Current Rheumatology Reports (2024)
-
Exosome inspired photo-triggered gelation hydrogel composite on modulating immune pathogenesis for treating rheumatoid arthritis
Journal of Nanobiotechnology (2023)
-
Network analyses reveal new insights into the effect of multicomponent Tr14 compared to single-component diclofenac in an acute inflammation model
Journal of Inflammation (2023)