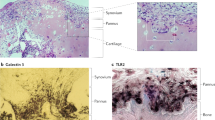
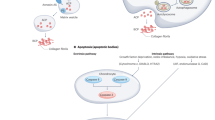
Abstract
Osteoarthritis (OA) is a progressive degenerative disease resulting in joint deterioration. Synovial inflammation is present in the OA joint and has been associated with radiographic and pain progression. Several OA risk factors, including ageing, obesity, trauma and mechanical loading, play a role in OA pathogenesis, likely by modifying synovial biology. In addition, other factors, such as mitochondrial dysfunction, damage-associated molecular patterns, cytokines, metabolites and crystals in the synovium, activate synovial cells and mediate synovial inflammation. An understanding of the activated pathways that are involved in OA-related synovial inflammation could form the basis for the stratification of patients and the development of novel therapeutics. This Review focuses on the biology of the OA synovium, how the cells residing in or recruited to the synovium interact with each other, how they become activated, how they contribute to OA progression and their interplay with other joint structures.
Key points
-
Imaging studies suggest that synovial inflammation may be present in both early osteoarthritis (OA) and advanced-stage OA and is involved in the development and progression of OA.
-
Synovial cells coordinate the production of molecules that initiate and maintain synovial inflammation and contribute to cartilage damage during OA progression.
-
Diverse stimuli, including bioactive lipids, prostaglandins, tricarboxylic acid cycle intermediates, cytokines and damage-associated molecular patterns, as well as clinical factors such as obesity, ageing, trauma and excessive mechanical loading, regulate the production of pro-inflammatory and anti-inflammatory mediators by synovial cells.
-
There is a need for functional imaging and cellular and molecular studies, together with a more robust histological interpretation at different stages of OA, to better stratify patients with OA and understand the role of synovitis in OA onset and progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hunter, D. J., March, L. & Chew, M., Lancet Commission on Osteoarthritis. Osteoarthritis in 2020 and beyond — Authors’ reply. Lancet 397, 1060 (2021).
Deshpande, B. R. et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 68, 1743–1750 (2016).
Loeser, R. F., Collins, J. A. & Diekman, B. O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 412–420 (2016).
Felson, D. T. et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage 24, 458–464 (2016).
Collins, J. E. et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol. 68, 2422–2431 (2016).
Wang, Y. et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology 58, 246–253 (2019).
Atukorala, I. et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann. Rheum. Dis. 75, 390–395 (2016).
MacFarlane, L. A. et al. Association of changes in effusion-synovitis with progression of cartilage damage over eighteen months in patients with osteoarthritis and meniscal tear. Arthritis Rheumatol. 71, 73–81 (2019).
Ayral, X., Pickering, E. H., Woodworth, T. G., Mackillop, N. & Dougados, M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005).
Roemer, F. W. et al. Can structural joint damage measured with MR imaging be used to predict knee replacement in the following year? Radiology 274, 810–820 (2015).
Roemer, F. W. et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809 (2011).
Perry, T. A. et al. Association between bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthritis Cartilage 28, 316–323 (2020).
Haugen, I. K. et al. Synovitis and radiographic progression in non-erosive and erosive hand osteoarthritis: is erosive hand osteoarthritis a separate inflammatory phenotype? Osteoarthritis Cartilage 24, 647–654 (2016).
Haugen, I. K. et al. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann. Rheum. Dis. 75, 117–123 (2016).
Mancarella, L., Addimanda, O., Cavallari, C. & Meliconi, R. Synovial inflammation drives structural damage in hand osteoarthritis: a narrative literature review. Curr. Rheumatol. Rev. 13, 43–50 (2017).
Marshall, M., Watt, F. E., Vincent, T. L. & Dziedzic, K. Hand osteoarthritis: clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 14, 641–656 (2018).
Driban, J. B. et al. Risk factors and the natural history of accelerated knee osteoarthritis: a narrative review. BMC Musculoskelet. Disord. 21, 332 (2020).
Wyatt, L. A. et al. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthritis Cartilage 27, 667–675 (2019).
Sarmanova, A. et al. Association between ultrasound-detected synovitis and knee pain: a population-based case-control study with both cross-sectional and follow-up data. Arthritis Res. Ther. 19, 281 (2017).
Hill, C. L. et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 66, 1599–1603 (2007).
Riis, R. G. et al. The association between histological, macroscopic and magnetic resonance imaging assessed synovitis in end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 25, 272–280 (2017).
Abbasi, B., Pezeshki-Rad, M., Akhavan, R. & Sahebari, M. Association between clinical and sonographic synovitis in patients with painful knee osteoarthritis. Int. J. Rheum. Dis. 20, 561–566 (2017).
Baker, K. et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 69, 1779–1783 (2010).
Ballegaard, C. et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 22, 933–940 (2014).
Kanthawang, T. et al. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: data from the osteoarthritis initiative. Skeletal Radiol. 50, 217–229 (2021).
Guermazi, A. et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann. Rheum. Dis. 70, 805–811 (2011).
Riis, R. G. et al. Synovitis assessed on static and dynamic contrast-enhanced magnetic resonance imaging and its association with pain in knee osteoarthritis: a cross-sectional study. Eur. J. Radiol. 85, 1099–1108 (2016).
Bacon, K., LaValley, M. P., Jafarzadeh, S. R. & Felson, D. Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann. Rheum. Dis. 79, 1105–1110 (2020).
Chevalier, X., Eymard, F. & Richette, P. Biologic agents in osteoarthritis: hopes and disappointments. Nat. Rev. Rheumatol. 9, 400–410 (2013).
Ansari, M. Y. et al. Genetic inactivation of ZCCHC6 suppresses interleukin-6 expression and reduces the severity of experimental osteoarthritis in mice. Arthritis Rheumatol. 71, 583–593 (2019).
Attur, M. et al. Interleukin 1 receptor antagonist (IL1RN) gene variants predict radiographic severity of knee osteoarthritis and risk of incident disease. Ann. Rheum. Dis. 79, 400–407 (2020).
Latourte, A. et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 76, 748–755 (2017).
Nasi, S., So, A., Combes, C., Daudon, M. & Busso, N. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann. Rheum. Dis. 75, 1372–1379 (2016).
Kadri, A. et al. Osteoprotegerin inhibits cartilage degradation through an effect on trabecular bone in murine experimental osteoarthritis. Arthritis Rheum. 58, 2379–2386 (2008).
Chevalier, X. et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 74, 1697–1705 (2015).
Kloppenburg, M. et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1alpha and anti-interleukin-1beta dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 78, 413–420 (2019).
Persson, M. S. M., Sarmanova, A., Doherty, M. & Zhang, W. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology 57, 1830–1837 (2018).
Richette, P. et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2020-218547 (2020).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Chao, J. et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J. Rheumatol. 37, 650–655 (2010).
de Lange-Brokaar, B. J. et al. Degree of synovitis on MRI by comprehensive whole knee semi-quantitative scoring method correlates with histologic and macroscopic features of synovial tissue inflammation in knee osteoarthritis. Osteoarthritis Cartilage 22, 1606–1613 (2014).
Loeuille, D. et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 52, 3492–3501 (2005).
af Klint, E. et al. Evaluation of arthroscopy and macroscopic scoring. Arthritis Res. Ther. 11, R81 (2009).
Krenn, V. et al. Grading of chronic synovitis — a histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract. 198, 317–325 (2002).
Krenn, V. et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49, 358–364 (2006).
Slansky, E. et al. Quantitative determination of the diagnostic accuracy of the synovitis score and its components. Histopathology 57, 436–443 (2010).
Krenn, V. et al. 15 years of the histopathological synovitis score, further development and review: a diagnostic score for rheumatology and orthopaedics. Pathol. Res. Pract. 213, 874–881 (2017).
Oehler, S., Neureiter, D., Meyer-Scholten, C. & Aigner, T. Subtyping of osteoarthritic synoviopathy. Clin. Exp. Rheumatol. 20, 633–640 (2002).
Mathiessen, A. & Conaghan, P. G. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res. Ther. 19, 18 (2017).
Mussawy, H. et al. The histopathological synovitis score is influenced by biopsy location in patients with knee osteoarthritis. Arch. Orthop. Trauma. Surg. https://doi.org/10.1007/s00402-021-03889-x (2021).
de Lange-Brokaar, B. J. et al. Association of pain in knee osteoarthritis with distinct patterns of synovitis. Arthritis Rheumatol. 67, 733–740 (2015).
Nanus, D. E. et al. Synovial tissue from sites of joint pain in knee osteoarthritis patients exhibits a differential phenotype with distinct fibroblast subsets. EBioMedicine https://doi.org/10.1016/j.ebiom.2021.103618 (2021).
Wyatt, L. A. et al. Histopathological subgroups in knee osteoarthritis. Osteoarthritis Cartilage 25, 14–22 (2017).
Benito, M. J., Veale, D. J., FitzGerald, O., van den Berg, W. B. & Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267 (2005).
Livshits, G. et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford Study. Arthritis Rheum. 60, 2037–2045 (2009).
Stannus, O. et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage 18, 1441–1447 (2010).
Labinsky, H. et al. Multiparameter analysis identifies heterogeneity in knee osteoarthritis synovial responses. Arthritis Rheumatol. 72, 598–608 (2020).
Chou, C. H. et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 10, 10868 (2020).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Klein-Wieringa, I. R. et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J. Rheumatol. 43, 771–778 (2016).
Wood, M. J. et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 4, e125325 (2019).
Watanabe, S., Alexander, M., Misharin, A. V. & Budinger, G. R. S. The role of macrophages in the resolution of inflammation. J. Clin. Invest. 129, 2619–2628 (2019).
Gomez-Aristizabal, A., Gandhi, R., Mahomed, N. N., Marshall, K. W. & Viswanathan, S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study. Arthritis Res. Ther. 21, 26 (2019).
Kraus, V. B. et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage 24, 1613–1621 (2016).
Raghu, H. et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 76, 914–922 (2017).
Bondeson, J. et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62, 647–657 (2010).
Bondeson, J., Wainwright, S. D., Lauder, S., Amos, N. & Hughes, C. E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 8, R187 (2006).
Wu, C. L. et al. Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage fas-induced apoptosis-transgenic mice. Arthritis Rheumatol. 69, 1772–1783 (2017).
Orecchioni, M., Ghosheh, Y., Pramod, A. B. & Ley, K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front. Immunol. 10, 1084 (2019).
Viola, A., Munari, F., Sanchez-Rodriguez, R., Scolaro, T. & Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 10, 1462 (2019).
Daghestani, H. N., Pieper, C. F. & Kraus, V. B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 67, 956–965 (2015).
Liu, B., Zhang, M., Zhao, J., Zheng, M. & Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 16, 5009–5014 (2018).
Manferdini, C. et al. From osteoarthritic synovium to synovial-derived cells characterization: synovial macrophages are key effector cells. Arthritis Res. Ther. 18, 83 (2016).
Fahy, N. et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage 22, 1167–1175 (2014).
Saito, I., Koshino, T., Nakashima, K., Uesugi, M. & Saito, T. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage 10, 156–162 (2002).
Madsen, D. H. et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 202, 951–966 (2013).
Dai, M., Sui, B., Xue, Y., Liu, X. & Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 180, 91–103 (2018).
Hoeksema, M. A. & Glass, C. K. Nature and nurture of tissue-specific macrophage phenotypes. Atherosclerosis 281, 159–167 (2019).
Young, L. et al. Effects of intraarticular glucocorticoids on macrophage infiltration and mediators of joint damage in osteoarthritis synovial membranes: findings in a double-blind, placebo-controlled study. Arthritis Rheum. 44, 343–350 (2001).
Siebelt, M. et al. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res. Ther. 17, 352 (2015).
Ha, C. W. et al. A multicenter, single-blind, phase IIa clinical trial to evaluate the efficacy and safety of a cell-mediated gene therapy in degenerative knee arthritis patients. Hum. Gene Ther. Clin. Dev. 26, 125–130 (2015).
Cherian, J. J. et al. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-beta1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage 23, 2109–2118 (2015).
Ting, R. et al. Fast 18F labeling of a near-infrared fluorophore enables positron emission tomography and optical imaging of sentinel lymph nodes. Bioconjug. Chem. 21, 1811–1819 (2010).
Yang, X. et al. Detection of osteoarthritis inflammation by single-photon emission computed tomography based on an inflammation-targeting peptide cFLFLF. Mol. Imaging Biol. 23, 895–904 (2021).
Meester, E. J. et al. Imaging inflammation in atherosclerotic plaques, targeting SST2 with [111In]In-DOTA-JR11. J. Nucl. Cardiol. 28, 2506–2513 (2021).
Temple-Wong, M. M. et al. Hyaluronan concentration and size distribution in human knee synovial fluid: variations with age and cartilage degeneration. Arthritis Res. Ther. 18, 18 (2016).
Balazs, E. A. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg. Technol. Int. 12, 278–289 (2004).
Ni, S. et al. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-kappaB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res. Ther. 17, 91 (2015).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J. P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Hsueh, M. F., Zhang, X., Wellman, S. S., Bolognesi, M. P. & Kraus, V. B. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol. 73, 89–99 (2021).
Gupta, K., Shukla, M., Cowland, J. B., Malemud, C. J. & Haqqi, T. M. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 56, 3326–3335 (2007).
de Lange-Brokaar, B. J. et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage 24, 664–671 (2016).
Wang, Q. et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. eLife 8, e39905 (2019).
Sousa-Valente, J. et al. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthritis Cartilage 26, 84–94 (2018).
Enomoto, H. et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am. J. Pathol. 162, 171–181 (2003).
Kennedy, A. et al. Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum. 62, 711–721 (2010).
Kim, H. R., Lee, J. H., Kim, K. W., Kim, B. M. & Lee, S. H. The relationship between synovial fluid VEGF and serum leptin with ultrasonographic findings in knee osteoarthritis. Int. J. Rheum. Dis. 19, 233–240 (2016).
Nagao, M. et al. Vascular endothelial growth factor in cartilage development and osteoarthritis. Sci. Rep. 7, 13027 (2017).
Li, Y. S., Luo, W., Zhu, S. A. & Lei, G. H. T cells in osteoarthritis: alterations and beyond. Front. Immunol. 8, 356 (2017).
Rosshirt, N. et al. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther. 23, 37 (2021).
Nees, T. A. et al. T helper cell infiltration in osteoarthritis-related knee pain and disability. J. Clin. Med. 9, 2423 (2020).
Thomas, A. C., Hubbard-Turner, T., Wikstrom, E. A. & Palmieri-Smith, R. M. Epidemiology of posttraumatic osteoarthritis. J. Athl. Train. 52, 491–496 (2017).
Whittaker, J. L. et al. Association between MRI-defined osteoarthritis, pain, function and strength 3–10 years following knee joint injury in youth sport. Br. J. Sports Med. 52, 934–939 (2018).
Scanzello, C. R. et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63, 391–400 (2011).
Pessler, F. et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann. Rheum. Dis. 67, 1184–1187 (2008).
Lieberthal, J., Sambamurthy, N. & Scanzello, C. R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 23, 1825–1834 (2015).
Davis, J. E. et al. A single recent injury is a potent risk factor for the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol. Int. 37, 1759–1764 (2017).
Driban, J. B. et al. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res. 66, 1673–1679 (2014).
Gelber, A. C. et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 133, 321–328 (2000).
Sward, P., Frobell, R., Englund, M., Roos, H. & Struglics, A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis) — a cross-sectional analysis. Osteoarthritis Cartilage 20, 1302–1308 (2012).
Pham, T. M., Erichsen, J. L., Kowal, J. M., Overgaard, S. & Schmal, H. Elevation of pro-inflammatory cytokine levels following intra-articular fractures-a systematic review. Cells 10, 902 (2021).
Pham, T. M., Frich, L. H., Lambertsen, K. L., Overgaard, S. & Schmal, H. Elevation of inflammatory cytokines and proteins after intra-articular ankle fracture: a cross-sectional study of 47 ankle fracture patients. Mediators Inflamm. 2021, 8897440 (2021).
Jacobs, C. A. et al. Dysregulated inflammatory response related to cartilage degradation after ACL injury. Med. Sci. Sports Exerc. 52, 535–541 (2020).
Adams, S. B. et al. Inflammatory microenvironment persists after bone healing in intra-articular ankle fractures. Foot Ankle Int. 38, 479–484 (2017).
Roemer, F. W. et al. Molecular and structural biomarkers of inflammation at two years after acute anterior cruciate ligament injury do not predict structural knee osteoarthritis at five years. Arthritis Rheumatol. 71, 238–243 (2019).
Griffin, T. M. & Guilak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport. Sci. Rev. 33, 195–200 (2005).
Fang, T., Zhou, X., Jin, M., Nie, J. & Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 45, 1125–1136 (2021).
Nazet, U. et al. Housekeeping gene validation for RT-qPCR studies on synovial fibroblasts derived from healthy and osteoarthritic patients with focus on mechanical loading. PLoS One 14, e0225790 (2019).
Fahy, N., Menzel, U., Alini, M. & Stoddart, M. J. Shear and dynamic compression modulates the inflammatory phenotype of human monocytes in vitro. Front. Immunol. 10, 383 (2019).
Ruffino, J. S. et al. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: a putative role for PPARgamma. Eur. J. Appl. Physiol. 116, 1671–1682 (2016).
Helmark, I. C. et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res. Ther. 12, R126 (2010).
Boonstra, A. et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J. Immunol. 177, 7551–7558 (2006).
Yoshimura, N. et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 20, 1217–1226 (2012).
Batushansky, A. et al. Fundamentals of OA. An initiative of osteoarthritis and cartilage. Chapter 9: obesity and metabolic factors in OA. Osteoarthritis Cartilage https://doi.org/10.1016/j.joca.2021.06.013 (2021).
Prieto-Alhambra, D. et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 73, 1659–1664 (2014).
Losina, E. et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 65, 703–711 (2013).
Murphy, L. B. et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage 18, 1372–1379 (2010).
Berenbaum, F., Wallace, I. J., Lieberman, D. E. & Felson, D. T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 14, 674–681 (2018).
Harasymowicz, N. S. et al. Regional differences between perisynovial and infrapatellar adipose tissue depots and their response to Class II and Class III obesity in patients with osteoarthritis. Arthritis Rheumatol. 69, 1396–1406 (2017).
Duan, L. et al. Infrapatellar fat pads participate in the development of knee osteoarthritis in obese patients via the activation of the NFkappaB signaling pathway. Int. J. Mol. Med. 46, 2260–2270 (2020).
Takata, K. et al. Increase in tryptase and its role in the synovial membrane of overweight and obese patients with osteoarthritis of the knee. Diabetes Metab. Syndr. Obes. 13, 1491–1497 (2020).
Sun, A. R. et al. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS One 12, e0183693 (2017).
Larranaga-Vera, A. et al. Increased synovial lipodystrophy induced by high fat diet aggravates synovitis in experimental osteoarthritis. Arthritis Res. Ther. 19, 264 (2017).
Yusuf, E. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann. Rheum. Dis. 69, 761–765 (2010).
Reyes, C. et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. 68, 1869–1875 (2016).
Yang, W. H. et al. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PLoS One 8, e75551 (2013).
Jafarzadeh, S. R. et al. Mediating role of bone marrow lesions, synovitis, pain sensitization, and depressive symptoms on knee pain improvement following substantial weight loss. Arthritis Rheumatol. 72, 420–427 (2020).
Gudbergsen, H. et al. Changes in bone marrow lesions in response to weight-loss in obese knee osteoarthritis patients: a prospective cohort study. BMC Musculoskelet. Disord. 14, 106 (2013).
Henriksen, M. et al. Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage 22, 639–646 (2014).
Daugaard, C. L. et al. The impact of a significant weight loss on inflammation assessed on DCE-MRI and static MRI in knee osteoarthritis: a prospective cohort study. Osteoarthritis Cartilage 28, 766–773 (2020).
Piva, S. R. et al. Links between osteoarthritis and diabetes: implications for management from a physical activity perspective. Clin. Geriatr. Med. 31, 67–87 (2015).
Schett, G. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 36, 403–409 (2013).
Louati, K., Vidal, C., Berenbaum, F. & Sellam, J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 1, e000077 (2015).
Niu, J., Clancy, M., Aliabadi, P., Vasan, R. & Felson, D. T. Metabolic syndrome, its components, and knee osteoarthritis: the Framingham Osteoarthritis Study. Arthritis Rheumatol. 69, 1194–1203 (2017).
Monira Hussain, S. et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin. Arthritis Rheum. 43, 429–436 (2014).
Kuusalo, L. et al. Metabolic osteoarthritis — relation of diabetes and cardiovascular disease with knee osteoarthritis. Osteoarthritis Cartilage 29, 230–234 (2021).
Rogers-Soeder, T. S. et al. Association of diabetes mellitus and biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis. Arthritis Care Res. 72, 98–106 (2020).
Hamada, D. et al. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheumatol. 68, 1392–1402 (2016).
Griffin, T. M. & Huffman, K. M. Editorial: insulin resistance: releasing the brakes on synovial inflammation and osteoarthritis? Arthritis Rheumatol. 68, 1330–1333 (2016).
Tsai, C. H. et al. High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim. Biophys. Acta 1830, 2649–2658 (2013).
Steenvoorden, M. M. et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 54, 253–263 (2006).
Veronese, N. et al. The relationship between the dietary inflammatory index and prevalence of radiographic symptomatic osteoarthritis: data from the Osteoarthritis Initiative. Eur. J. Nutr. 58, 253–260 (2019).
Wang, X. et al. Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthritis Cartilage 25, 1304–1312 (2017).
Perry, T. A. et al. Effect of Vitamin D supplementation on synovial tissue volume and subchondral bone marrow lesion volume in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 20, 76 (2019).
Wang, Z. et al. Effectiveness of curcuma longa extract for the treatment of symptoms and effusion-synovitis of knee osteoarthritis: a randomized trial. Ann. Intern. Med. 173, 861–869 (2020).
Veronese, N. et al. Mediterranean diet and knee osteoarthritis outcomes: a longitudinal cohort study. Clin. Nutr. 38, 2735–2739 (2019).
Xu, C. et al. Dietary patterns and progression of knee osteoarthritis: data from the osteoarthritis initiative. Am. J. Clin. Nutr. 111, 667–676 (2020).
Ulici, V. et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthritis Cartilage 26, 1098–1109 (2018).
Dunn, C. M. et al. Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis Rheumatol. 72, 1111–1122 (2020).
Zhao, Y. et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 8, 14305 (2018).
Schott, E. M. et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 3, e95997 (2018).
Huang, Z. Y., Stabler, T., Pei, F. X. & Kraus, V. B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage 24, 1769–1775 (2016).
Mohammad, S. & Thiemermann, C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol. 11, 594150 (2020).
Fuke, N., Nagata, N., Suganuma, H. & Ota, T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 11, 2277 (2019).
Boer, C. G. et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 10, 4881 (2019).
Mao, X., Fu, P., Wang, L. & Xiang, C. Mitochondria: potential targets for osteoarthritis. Front. Med. 7, 581402 (2020).
Geurts, J. et al. Prematurely aging mitochondrial DNA mutator mice display subchondral osteopenia and chondrocyte hypertrophy without further osteoarthritis features. Sci. Rep. 10, 1296 (2020).
Torroni, A. et al. Classification of European mtDNAs from an analysis of three European populations. Genetics 144, 1835–1850 (1996).
Da Sylva, T. R., Connor, A., Mburu, Y., Keystone, E. & Wu, G. E. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res. Ther. 7, R844–R851 (2005).
Van den Bossche, J. et al. mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 17, 684–696 (2016).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Yohn, C. O. B., et al. Senescent synoviocytes in knee osteoarthritis correlate with disease biomarkers, synovitis, and knee pain [abstract]. Arthritis Rheumatol. https://acrabstracts.org/abstract/senescent-synoviocytes-in-knee-osteoarthritis-correlate-with-disease-biomarkers-synovitis-and-knee-pain/ (2019).
Anderson, J. R. et al. 1H NMR metabolomics identifies underlying inflammatory pathology in osteoarthritis and rheumatoid arthritis synovial joints. J. Proteome Res. 17, 3780–3790 (2018).
Farrell, A. J., Blake, D. R., Palmer, R. M. & Moncada, S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 51, 1219–1222 (1992).
Melchiorri, C. et al. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 41, 2165–2174 (1998).
Abramson, S. B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 10, S2 (2008).
Pelletier, J. P. et al. Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J. Rheumatol. 26, 2002–2014 (1999).
Hellio le Graverand, M. P. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 72, 187–195 (2013).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013).
Keiran, N. et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 20, 581–592 (2019).
Li, Y. et al. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1alpha/VEGF axis. Free Radic. Biol. Med. 126, 1–14 (2018).
Wojdasiewicz, P., Poniatowski, L. A. & Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 561459 (2014).
Futani, H. et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J. Immunother. 25, S61–S64 (2002).
Attur, M. et al. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J. Immunol. 181, 5082–5088 (2008).
Valdes, A. M. et al. Omega-6 oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J. Lipid Res. 59, 1763–1770 (2018).
Lambert, C. et al. The damage-associated molecular patterns (DAMPs) as potential targets to treat osteoarthritis: perspectives from a review of the literature. Front. Med. 7, 607186 (2020).
Millerand, M., Berenbaum, F. & Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 37, 48–56 (2019).
Heinola, T. et al. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin. Exp. Rheumatol. 28, 511–518 (2010).
Li, Z. C. et al. Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin. Invest. Med. 34, E298 (2011).
Ke, X. et al. Synovial fluid HMGB-1 levels are associated with osteoarthritis severity. Clin. Lab. 61, 809–818 (2015).
Aulin, C., Lassacher, T., Palmblad, K. & Erlandsson Harris, H. Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthritis Cartilage 28, 698–707 (2020).
Lambrecht, S., Juchtmans, N. & Elewaut, D. Heat-shock proteins in stromal joint tissues: innocent bystanders or disease-initiating proteins? Rheumatology 53, 223–232 (2014).
Takahashi, K. et al. Localization of heat shock protein in osteoarthritic cartilage. Scand. J. Rheumatol. 26, 368–375 (1997).
Ngarmukos, S., Scaramuzza, S., Theerawattanapong, N., Tanavalee, A. & Honsawek, S. Circulating and synovial fluid heat shock protein 70 are correlated with severity in knee osteoarthritis. Cartilage 11, 323–328 (2020).
Son, Y. O., Kim, H. E., Choi, W. S., Chun, C. H. & Chun, J. S. RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nat. Commun. 10, 77 (2019).
Lambert, C. et al. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 66, 960–968 (2014).
Schelbergen, R. F. et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann. Rheum. Dis. 75, 218–225 (2016).
Ruan, G. et al. Associations between serum S100A8/S100A9 and knee symptoms, joint structures and cartilage enzymes in patients with knee osteoarthritis. Osteoarthritis Cartilage 27, 99–105 (2019).
Addimanda, O. et al. Elevated serum levels of alarmin S100A8/A9 in patients with hand osteoarthritis. Clin. Exp. Rheumatol. 37, 885 (2019).
Daheshia, M. & Yao, J. Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 35, 2306–2312 (2008).
Inoue, H. et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone 42, 1102–1110 (2008).
McAllister, M. J., Chemaly, M., Eakin, A. J., Gibson, D. S. & McGilligan, V. E. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthritis Cartilage 26, 612–619 (2018).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Zhong, Z. et al. NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 (2016).
Sanchez-Lopez, E. et al. Choline uptake and metabolism modulate macrophage IL-1beta and IL-18 production. Cell Metab. 29, 1350–1362.e7 (2019).
Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018).
Jin, C. et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl Acad. Sci. USA 108, 14867–14872 (2011).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Denoble, A. E. et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl Acad. Sci. USA 108, 2088–2093 (2011).
Elsaid, K. A. et al. The impact of early intra-articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthritis Cartilage 23, 114–121 (2015).
Schieker, M. et al. Effects of interleukin-1beta inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 173, 509–515 (2020).
Lane, N. & Felson, D. A promising treatment for osteoarthritis? Ann. Intern. Med. 173, 580–581 (2020).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009).
Fu, Z. et al. Interleukin-18-induced inflammatory responses in synoviocytes and chondrocytes from osteoarthritic patients. Int. J. Mol. Med. 30, 805–810 (2012).
Shi, J., Gao, W. & Shao, F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 (2017).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Assirelli, E. et al. Complement expression and activation in osteoarthritis joint compartments. Front. Immunol. 11, 535010 (2020).
Li, Z., Huang, Z. & Bai, L. Cell interplay in osteoarthritis. Front. Cell Dev. Biol. 9, 720477 (2021).
Dreier, R., Wallace, S., Fuchs, S., Bruckner, P. & Grassel, S. Paracrine interactions of chondrocytes and macrophages in cartilage degradation: articular chondrocytes provide factors that activate macrophage-derived pro-gelatinase B (pro-MMP-9). J. Cell Sci. 114, 3813–3822 (2001).
Hamasaki, M. et al. Transcriptional profiling of murine macrophages stimulated with cartilage fragments revealed a strategy for treatment of progressive osteoarthritis. Sci. Rep. 10, 7558 (2020).
Silverstein, A. M. et al. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthritis Cartilage 25, 1353–1361 (2017).
Roemer, F. W. et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol. 67, 2085–2096 (2015).
Hugle, T. & Geurts, J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology 56, 1461–1471 (2017).
Hu, W., Chen, Y., Dou, C. & Dong, S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 80, 413–422 (2020).
Yang, C. C., Lin, C. Y., Wang, H. S. & Lyu, S. R. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS One 8, e79662 (2013).
Furuzawa-Carballeda, J., Macip-Rodriguez, P. M. & Cabral, A. R. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin. Exp. Rheumatol. 26, 554–560 (2008).
Yusup, A. et al. Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 23, 1858–1864 (2015).
Aso, K. et al. Associations of symptomatic knee osteoarthritis with histopathologic features in subchondral bone. Arthritis Rheumatol. 71, 916–924 (2019).
Arepati, A. I. et al. Osteophyte formation is associated with synovitis in osteoarthritis — the Bunkyo Health Study. Osteoarthritis Cartilage 28, S86–S527 (2020).
Blom, A. B. et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 12, 627–635 (2004).
van Lent, P. L. et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 50, 103–111 (2004).
Blaney Davidson, E. N. et al. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor beta-induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 56, 4065–4073 (2007).
Remst, D. F., Blaney Davidson, E. N. & van der Kraan, P. M. Unravelling osteoarthritis-related synovial fibrosis: a step closer to solving joint stiffness. Rheumatology 54, 1954–1963 (2015).
van Helvoort, E. M., Eijkelkamp, N., Lafeber, F. & Mastbergen, S. C. Expression of granulocyte macrophage-colony stimulating factor and its receptor in the synovium of osteoarthritis patients is negatively correlated with pain. Rheumatology 59, 3452–3457 (2020).
Wise, B. L., Seidel, M. F. & Lane, N. E. The evolution of nerve growth factor inhibition in clinical medicine. Nat. Rev. Rheumatol. 17, 34–46 (2021).
Stoppiello, L. A. et al. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 66, 3018–3027 (2014).
Cohen, S. B. et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 13, R125 (2011).
Vincent, T. L. Peripheral pain mechanisms in osteoarthritis. Pain 161, S138–S146 (2020).
Bratus-Neuenschwander, A. et al. Pain-associated transcriptome changes in synovium of knee osteoarthritis patients. Genes (Basel) 9, 338 (2018).
Attard, V. et al. Quantification of intra-articular fibrosis in patients with stiff knee arthroplasties using metal-reduction MRI. Bone Jt. J. 102-B, 1331–1340 (2020).
Kalson, N. S. et al. International consensus on the definition and classification of fibrosis of the knee joint. Bone Jt. J. 98-B, 1479–1488 (2016).
Toms, J. et al. Targeting fibroblast activation protein: radiosynthesis and preclinical evaluation of an 18F-labeled FAP inhibitor. J. Nucl. Med. 61, 1806–1813 (2020).
Kerna, I. et al. The ADAM12 is upregulated in synovitis and postinflammatory fibrosis of the synovial membrane in patients with early radiographic osteoarthritis. Jt. Bone Spine 81, 51–56 (2014).
Remst, D. F. et al. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor beta-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 66, 647–656 (2014).
Rim, Y. A. & Ju, J. H. The role of fibrosis in osteoarthritis progression. Life (Basel) 11, 3 (2020).
Bastiaansen-Jenniskens, Y. M. et al. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2alpha. Arthritis Rheum. 65, 2070–2080 (2013).
Eymard, F. et al. Knee and hip intra-articular adipose tissues (IAATs) compared with autologous subcutaneous adipose tissue: a specific phenotype for a central player in osteoarthritis. Ann. Rheum. Dis. 76, 1142–1148 (2017).
Davis, J. E. et al. Effusion-synovitis and infrapatellar fat pad signal intensity alteration differentiate accelerated knee osteoarthritis. Rheumatology 58, 418–426 (2019).
Inomata, K. et al. Time course analyses of structural changes in the infrapatellar fat pad and synovial membrane during inflammation-induced persistent pain development in rat knee joint. BMC Musculoskelet. Disord. 20, 8 (2019).
Barboza, E. et al. Profibrotic infrapatellar fat pad remodeling without M1 macrophage polarization precedes knee osteoarthritis in mice with diet-induced obesity. Arthritis Rheumatol. 69, 1221–1232 (2017).
de Jong, A. J. et al. Lack of high BMI-related features in adipocytes and inflammatory cells in the infrapatellar fat pad (IFP). Arthritis Res. Ther. 19, 186 (2017).
Warmink, K. et al. High-fat feeding primes the mouse knee joint to develop osteoarthritis and pathologic infrapatellar fat pad changes after surgically induced injury. Osteoarthritis Cartilage 28, 593–602 (2020).
Takano, S. et al. Transforming growth factor-beta stimulates nerve growth factor production in osteoarthritic synovium. BMC Musculoskelet. Disord. 20, 204 (2019).
Blaney Davidson, E. N. et al. TGF-beta is a potent inducer of nerve growth factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis Cartilage 3, 478–486 (2015).
Grässel, S. & Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Res. 9, F1000 (2020).
Vincent, T. L. Of mice and men: converging on a common molecular understanding of osteoarthritis. Lancet Rheumatol. 2, e633–e645 (2020).
Wang, X., Hunter, D. J., Jin, X. & Ding, C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthritis Cartilage 26, 165–174 (2018).
Roemer, F. W. et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage 18, 1269–1274 (2010).
Shakoor, D. et al. Are contrast-enhanced and non-contrast MRI findings reflecting synovial inflammation in knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 28, 126–136 (2020).
Takase, K. et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin. Exp. Rheumatol. 30, 85–92 (2012).
Walther, M. et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 44, 331–338 (2001).
Tarhan, S. & Unlu, Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin. Rheumatol. 22, 181–188 (2003).
Acknowledgements
The authors’ work was supported by the US National Institutes of Health (AR073324 to M.G., 5T32AR064194-07 to R.C., NIH Diversity Supplement to A.T., AG070647 and AR078917 to N.E.L., and K01AR077111 and Resource-based Center for the study of the joint microenvironment in rheumatology UCSD (NIH P30AR073761) to E.S.-L.).
Author information
Authors and Affiliations
Contributions
E.S.-L., R.C., N.E.L. and M.G. researched data for the article and contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks M. Wood and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanchez-Lopez, E., Coras, R., Torres, A. et al. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol 18, 258–275 (2022). https://doi.org/10.1038/s41584-022-00749-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00749-9
This article is cited by
-
Effect of weight loss on knee joint synovitis over 48 months and mediation by subcutaneous fat around the knee: data from the Osteoarthritis Initiative
BMC Musculoskeletal Disorders (2024)
-
ADAM8 silencing suppresses the migration and invasion of fibroblast-like synoviocytes via FSCN1/MAPK cascade in osteoarthritis
Arthritis Research & Therapy (2024)
-
Inhibition of the MALT1-LPCAT3 axis protects cartilage degeneration and osteoarthritis
Cell Communication and Signaling (2024)
-
M2 macrophage-derived exosomal miR-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting TLR3 and COL10A1 to alleviate osteoarthritis
Journal of Nanobiotechnology (2024)
-
Mechanistic role of quercetin as inhibitor for adenosine deaminase enzyme in rheumatoid arthritis: systematic review
Cellular & Molecular Biology Letters (2024)