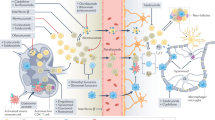
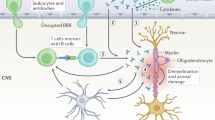
Abstract
Patients with systemic lupus erythematosus (SLE) frequently show symptoms of central nervous system (CNS) involvement, termed neuropsychiatric SLE (NPSLE). The CNS manifestations of SLE are diverse and have a broad spectrum of severity and prognostic implications. Patients with NPSLE typically present with nonspecific symptoms, such as headache and cognitive impairment, but might also experience devastating features, such as memory loss, seizures and stroke. Some features of NPSLE, in particular those related to coagulopathy, have been characterized and an evidence-based treatment algorithm is available. The cognitive and affective manifestations of NPSLE, however, remain poorly understood. Various immune effectors have been evaluated as contributors to its pathogenesis, including brain-reactive autoantibodies, cytokines and cell-mediated inflammation. Additional brain-intrinsic elements (such as resident microglia, the blood–brain barrier and other neurovascular interfaces) are important facilitators of NPSLE. As yet, however, no unifying model has been found to underlie the pathogenesis of NPSLE, suggesting that this disease has multiple contributors and perhaps several distinct aetiologies. This heterogeneity presents a challenge for clinicians who have traditionally relied on empirical judgement in choosing treatment modalities for patients with NPSLE. Improved understanding of this manifestation of SLE might yield further options for managing this disease.
Key points
-
Management of neuropsychiatric symptoms in patients with systemic lupus erythematosus (SLE) remains challenging as evidence-based regimens are not generally available.
-
A pressing need in the management of neuropsychiatric SLE (NPSLE) is the appropriate attribution of symptoms to either primary inflammatory pathology or secondary consequences of the general SLE disease burden.
-
Research efforts are aggressively pursuing the identification of pathways involved in NPSLE development, along with new therapeutic targets.
-
Mechanisms at the neuroimmune interface are being studied and might extend beyond the cerebral circulation and the blood–brain barrier to include the blood–cerebrospinal fluid barrier and/or the meningeal barrier.
-
Novel therapies, including small-molecule inhibitors and biologic agents that target inflammatory pathways, are currently being explored to target NPSLE specifically.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Unterman, A. et al. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin. Arthritis Rheum. 41, 1–11 (2011).
Ainiala, H., Loukkola, J., Peltola, J., Korpela, M. & Hietaharju, A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 57, 496–500 (2001).
Bertsias, G. K. & Boumpas, D. T. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat. Rev. Rheumatol. 6, 358–367 (2010).
Borowoy, A. M. et al. Neuropsychiatric lupus: the prevalence and autoantibody associations depend on the definition: results from the 1000 Faces of Lupus cohort. Semin. Arthritis Rheum. 42, 179–185 (2012).
Kozora, E. et al. Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus 21, 402–411 (2012).
The American College of Rheumatology. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 42, 599–608 (1999).
Hanly, J. G. et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 69, 529–535 (2010).
Mok, C. C., Lau, C. S. & Wong, R. W. Neuropsychiatric manifestations and their clinical associations in southern Chinese patients with systemic lupus erythematosus. J. Rheumatol. 28, 766–771 (2001).
Ho, R. C. et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun. Rev. 15, 124–138 (2016).
Steup-Beekman, G. M. et al. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: epidemiology and radiology pointing to an immune-mediated cause. Ann. Rheum. Dis. 72 (Suppl. 2), ii76–ii79 (2013).
Bujan, S. et al. Contribution of the initial features of systemic lupus erythematosus to the clinical evolution and survival of a cohort of Mediterranean patients. Ann. Rheum. Dis. 62, 859–865 (2003).
Govoni, M. et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 51, 157–168 (2012).
Govoni, M. et al. The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J. Autoimmun. 74, 41–72 (2016).
Hanly, J. G. et al. Cerebrovascular events in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Care Res. 70, 1478–1487 (2018).
Mikdashi, J. & Handwerger, B. Predictors of neuropsychiatric damage in systemic lupus erythematosus: data from the Maryland lupus cohort. Rheumatology (Oxford) 43, 1555–1560 (2004).
Brey, R. L., Gharavi, A. E. & Lockshin, M. D. Neurologic complications of antiphospholipid antibodies. Rheum. Dis. Clin. North Am. 19, 833–850 (1993).
Ellis, S. G. & Verity, M. A. Central nervous system involvement in systemic lupus erythematosus: a review of neuropathologic findings in 57 cases, 1955–1977. Semin. Arthritis Rheum. 8, 212–221 (1979).
Hanly, J. G. et al. Prospective study of neuropsychiatric events in systemic lupus erythematosus. J. Rheumatol. 36, 1449–1459 (2009).
Bertsias, G. K. et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann. Rheum. Dis. 69, 2074–2082 (2010).
Cohen, D. et al. Brain histopathology in patients with systemic lupus erythematosus: identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford) 56, 77–86 (2017).
Luyendijk, J. et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum. 63, 722–732 (2011).
Hanly, J. G., Kozora, E., Beyea, S. & Birnbaum, J. Nervous system disease in systemic lupus erythematosus: current status and future directions. Arthritis Rheumatol. 71, 33–42 (2018).
Bortoluzzi, A., Scire, C. A. & Govoni, M. Attribution of neuropsychiatric manifestations to systemic lupus erythematosus. Front. Med. 5, 68 (2018).
Hanly, J. G. et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 56, 265–273 (2007).
Hanly, J. G. et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum. 59, 721–729 (2008).
Bortoluzzi, A. et al. Development and validation of a new algorithm for attribution of neuropsychiatric events in systemic lupus erythematosus. Rheumatology (Oxford) 54, 891–898 (2015).
Magro-Checa, C. et al. Value of multidisciplinary reassessment in attribution of neuropsychiatric events to systemic lupus erythematosus: prospective data from the Leiden NPSLE cohort. Rheumatology (Oxford) 56, 1676–1683 (2017).
Castillo-Gomez, E. et al. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol. Psychiatry 22, 1776–1784 (2017).
Kowal, C. et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA 103, 19854–19859 (2006).
Bravo-Zehnder, M. et al. Anti-ribosomal P protein autoantibodies from patients with neuropsychiatric lupus impair memory in mice. Arthritis Rheumatol. 67, 204–214 (2015).
Du, Y., Sanam, S., Kate, K. & Mohan, C. Animal models of lupus and lupus nephritis. Curr. Pharm. Des. 21, 2320–2349 (2015).
Kier, A. B. Clinical neurology and brain histopathology in NZB/NZW F1 lupus mice. J. Comp. Pathol. 102, 165–177 (1990).
Leung, J. W., Lau, B. W., Chan, V. S., Lau, C. S. & So, K. F. Abnormal increase of neuronal precursor cells and exacerbated neuroinflammation in the corpus callosum in murine model of systemic lupus erythematosus. Restor. Neurol. Neurosci. 34, 443–453 (2016).
Ballok, D. A. Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res. Rev. 54, 67–79 (2007).
Williams, S., Stafford, P. & Hoffman, S. A. Diagnosis and early detection of CNS-SLE in MRL/lpr mice using peptide microarrays. BMC Immunol. 15, 23 (2014).
Han, J. H. et al. Expression of an anti-RNA autoantibody in a mouse model of SLE increases neutrophil and monocyte numbers as well as IFN-I expression. Eur. J. Immunol. 44, 215–226 (2014).
McDonald, G. et al. Accelerated systemic autoimmunity in the absence of somatic hypermutation in 564Igi: a mouse model of systemic lupus with knocked-in heavy and light chain genes. Front. Immunol. 8, 1094 (2017).
Bialas, A. R. et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature 546, 539–543 (2017).
Shi, D. et al. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav. Immun. 70, 293–304 (2018).
de Amorim, L. C., Maia, F. M. & Rodrigues, C. E. Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus 26, 529–536 (2017).
Sarbu, N. et al. Brain abnormalities in newly diagnosed neuropsychiatric lupus: systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort. Autoimmun. Rev. 14, 153–159 (2015).
Merali, Z., Huang, K., Mikulis, D., Silver, F. & Kassner, A. Evolution of blood–brain-barrier permeability after acute ischemic stroke. PLOS ONE 12, e0171558 (2017).
Kuntz, M. et al. Stroke-induced brain parenchymal injury drives blood–brain barrier early leakage kinetics: a combined in vivo/in vitro study. J. Cereb. Blood Flow Metab. 34, 95–107 (2014).
Knowland, D. et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82, 603–617 (2014).
Rochfort, K. D. & Cummins, P. M. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem. Soc. Trans. 43, 702–706 (2015).
Dimitrijevic, O. B., Stamatovic, S. M., Keep, R. F. & Andjelkovic, A. V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38, 1345–1353 (2007).
Yepes, M. et al. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am. J. Pathol. 166, 511–520 (2005).
Salahuddin, T. S., Kalimo, H., Johansson, B. B. & Olsson, Y. Observations on exudation of fibronectin, fibrinogen and albumin in the brain after carotid infusion of hyperosmolar solutions. An immunohistochemical study in the rat indicating long-lasting changes in the brain microenvironment and multifocal nerve cell injuries. Acta Neuropathol. 76, 1–10 (1988).
Wen, J. et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J. Autoimmun. 60, 40–50 (2015).
Fragoso-Loyo, H. et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLOS ONE 3, e3347 (2008).
Omdal, R. et al. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur. J. Neurol. 12, 392–398 (2005).
Hirohata, S., Arinuma, Y., Yanagida, T. & Yoshio, T. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res. Ther. 16, R77 (2014).
Hirohata, S., Sakuma, Y., Yanagida, T. & Yoshio, T. Association of cerebrospinal fluid anti-Sm antibodies with acute confusional state in systemic lupus erythematosus. Arthritis Res. Ther. 16, 450 (2014).
Bonfa, E. et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N. Engl. J. Med. 317, 265–271 (1987).
Schneebaum, A. B. et al. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am. J. Med. 90, 54–62 (1991).
Nojima, Y. et al. Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 51, 1053–1055 (1992).
Hammer, C. et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol. Psychiatry 19, 1143–1149 (2014).
Huerta, P. T., Kowal, C., DeGiorgio, L. A., Volpe, B. T. & Diamond, B. Immunity and behavior: antibodies alter emotion. Proc. Natl Acad. Sci. USA 103, 678–683 (2006).
Kowal, C. et al. Cognition and immunity; antibody impairs memory. Immunity 21, 179–188 (2004).
Lapteva, L. et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 54, 2505–2514 (2006).
Tumani, H., Huss, A. & Bachhuber, F. The cerebrospinal fluid and barriers - anatomic and physiologic considerations. Handb. Clin. Neurol. 146, 21–32 (2017).
Yang, L. et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 11, 107 (2013).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Baizabal-Carvallo, J. F., Delgadillo-Marquez, G., Estanol, B. & Garcia-Ramos, G. Clinical characteristics and outcomes of the meningitides in systemic lupus erythematosus. Eur. Neurol. 61, 143–148 (2009).
Kakati, S., Barman, B., Ahmed, S. U. & Hussain, M. Neurological manifestations in systemic lupus erythematosus: a single centre study from North East India. J. Clin. Diagn. Res. 11, OC05–OC09 (2017).
Yelehe-Okouma, M., Czmil-Garon, J., Pape, E., Petitpain, N. & Gillet, P. Drug-induced aseptic meningitis: a mini-review. Fund. Clin. Pharmacol. 32, 252–260 (2018).
van Veen, K. E. B., Brouwer, M. C., van der Ende, A. & van de Beek, D. Bacterial meningitis in patients using immunosuppressive medication: a population-based prospective nationwide study. J. Neuroimmune Pharmacol. 12, 213–218 (2017).
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523 (2009).
Baruch, K. et al. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014).
Atkins, C. J., Kondon, J. J., Quismorio, F. P. & Friou, G. J. The choroid plexus in systemic lupus erythematosus. Ann. Intern. Med. 76, 65–72 (1972).
Sher, J. H. & Pertschuk, L. P. Immunoglobulin G deposits in the choroid plexus of a child with systemic lupus erythematosus. J. Pediatr. 85, 385–387 (1974).
Gershwin, M. E., Hyman, L. R. & Steinberg, A. D. The choroid plexus in CNS involvement of systemic lupus erythematosus. J. Pediatr. 87, 588–590 (1975).
Boyer, R. S., Sun, N. C., Verity, A., Nies, K. M. & Louie, J. S. Immunoperoxidase staining of the choroid plexus in systemic lupus erythematosus. J. Rheumatol. 7, 645–650 (1980).
Amaro, E. Jr & Scheinberg, M. Onset of cognitive dysfunction in systemic lupus erythematosus and selective involvement of the choroid plexus. J. Rheumatol. 36, 2554–2555 (2009).
Stock, A., Wen, J., Doerner, J. & Putterman, C. Neuropsychiatric lupus occurs independently of systemic autoimmunity. J. Immunol. 194, 2 (2015).
James, W. G., Hutchinson, P., Bullard, D. C. & Hickey, M. J. Cerebral leucocyte infiltration in lupus-prone MRL/MpJ-fas lpr mice — roles of intercellular adhesion molecule-1 and P-selectin. Clin. Exp. Immunol. 144, 299–308 (2006).
Ma, X., Foster, J. & Sakic, B. Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J. Neuroimmunol. 179, 26–36 (2006).
Ballok, D. A., Ma, X., Denburg, J. A., Arsenault, L. & Sakic, B. Ibuprofen fails to prevent brain pathology in a model of neuropsychiatric lupus. J. Rheumatol. 33, 2199–2213 (2006).
Gelb, S., Stock, A. D., Anzi, S., Putterman, C. & Ben-Zvi, A. Mechanisms of neuropsychiatric lupus: the relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. J. Autoimmun. 91, 34–44 (2018).
Stock, A. D., Gelb, S., Pasternak, O., Ben-Zvi, A. & Putterman, C. The blood brain barrier and neuropsychiatric lupus: new perspectives in light of advances in understanding the neuroimmune interface. Autoimmun. Rev. 16, 612–619 (2017).
Schreiber, K. et al. Antiphospholipid syndrome. Nat. Rev. Dis. Primers 4, 18005 (2018).
Gao, C. et al. Thrombotic role of blood and endothelial cells in uremia through phosphatidylserine exposure and microparticle release. PLOS ONE 10, e0142835b (2015).
Giannakopoulos, B. & Krilis, S. A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 368, 1033–1044 (2013).
Narshi, C. B., Giles, I. P. & Rahman, A. The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus 20, 5–13 (2011).
Kittner, S. J. & Gorelick, P. B. Antiphospholipid antibodies and stroke: an epidemiological perspective. Stroke 23, I19–22 (1992).
Andrade, R. M. et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV). Ann. Rheum. Dis. 67, 829–834 (2008).
Appenzeller, S., Cendes, F. & Costallat, L. T. Epileptic seizures in systemic lupus erythematosus. Neurology 63, 1808–1812 (2004).
McLaurin, E. Y., Holliday, S. L., Williams, P. & Brey, R. L. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology 64, 297–303 (2005).
Mok, M. Y. et al. Antiphospholipid antibody profiles and their clinical associations in Chinese patients with systemic lupus erythematosus. J. Rheumatol. 32, 622–628 (2005).
Sanna, G. et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J. Rheumatol. 30, 985–992 (2003).
Tomietto, P. et al. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 57, 1461–1472 (2007).
Katzav, A. et al. Antibody-specific behavioral effects: intracerebroventricular injection of antiphospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J. Neuroimmunol. 272, 10–15 (2014).
Chi, O. Z., Hunter, C., Liu, X. & Weiss, H. R. Effects of exogenous excitatory amino acid neurotransmitters on blood-brain barrier disruption in focal cerebral ischemia. Neurochem. Res. 34, 1249–1254 (2009).
Du, H., Chen, M., Zhang, Y., Zhao, M. H. & Wang, H. Y. Cross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells in sera from patients with lupus nephritis. Clin. Exp. Immunol. 145, 21–27 (2006).
Zhao, Z. et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 52, 522–530 (2005).
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Faust, T. W. et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl Acad. Sci. USA 107, 18569–18574 (2010).
Gao, H. X. et al. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J. Neuroimmunol. 207, 45–56 (2009).
Arinuma, Y., Yanagida, T. & Hirohata, S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 58, 1130–1135 (2008).
Kozora, E. et al. Antibodies against N-methyl-D-aspartate receptors in patients with systemic lupus erythematosus without major neuropsychiatric syndromes. J. Neurol. Sci. 295, 87–91 (2010).
Petri, M. et al. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J. Rheumatol. 37, 2032–2038 (2010).
Brimberg, L. et al. Antibodies as mediators of brain pathology. Trends Immunol. 36, 709–724 (2015).
Husebye, E. S. et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 64, 1210–1213 (2005).
Nestor, J. et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J. Exp. Med. 215, 2554–2566 (2018).
Eber, T., Chapman, J. & Shoenfeld, Y. Anti-ribosomal P-protein and its role in psychiatric manifestations of systemic lupus erythematosus: myth or reality? Lupus 14, 571–575 (2005).
Tzioufas, A. G. et al. The clinical relevance of antibodies to ribosomal-P common epitope in two targeted systemic lupus erythematosus populations: a large cohort of consecutive patients and patients with active central nervous system disease. Ann. Rheum. Dis. 59, 99–104 (2000).
Moscavitch, S. D., Szyper-Kravitz, M. & Shoenfeld, Y. Autoimmune pathology accounts for common manifestations in a wide range of neuro-psychiatric disorders: the olfactory and immune system interrelationship. Clin. Immunol. 130, 235–243 (2009).
Yoshio, T. et al. Quantification of antiribosomal P0 protein antibodies by ELISA with recombinant P0 fusion protein and their association with central nervous system disease in systemic lupus erythematosus. J. Rheumatol. 22, 1681–1687 (1995).
Katzav, A. et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 56, 938–948 (2007).
Katzav, A. et al. Anti-P ribosomal antibodies induce defect in smell capability in a model of CNS-SLE (depression). J. Autoimmun. 31, 393–398 (2008).
Perricone, C. et al. Smell and autoimmunity: a comprehensive review. Clin. Rev. Allergy Immunol. 45, 87–96 (2013).
Song, C. & Leonard, B. E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 29, 627–647 (2005).
Elkon, K. B., Parnassa, A. P. & Foster, C. L. Lupus autoantibodies target ribosomal P proteins. J. Exp. Med. 162, 459–471 (1985).
Segovia-Miranda, F. et al. Pathogenicity of lupus anti-ribosomal P antibodies: role of cross-reacting neuronal surface P antigen in glutamatergic transmission and plasticity in a mouse model. Arthritis Rheumatol. 67, 1598–1610 (2015).
Nagai, T., Yanagida, T. & Hirohata, S. Anti-ribosomal P protein antibody induces Th1 responses by enhancing the production of IL-12 in activated monocytes. Mod. Rheumatol. 21, 57–62 (2011).
Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. & Hinson, S. R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Dellavance, A. et al. Anti-aquaporin-4 antibodies in the context of assorted immune-mediated diseases. Eur. J. Neurol. 19, 248–252 (2012).
Verkman, A. S., Phuan, P. W., Asavapanumas, N. & Tradtrantip, L. Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO. Brain Pathol. 23, 684–695 (2013).
Waters, P. et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch. Neurol. 65, 913–919 (2008).
Waters, P. J. et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 78, 665–671 (2012).
Mader, S. et al. Understanding the antibody repertoire in neuropsychiatric systemic lupus erythematosus and neuromyelitis optica spectrum disorder: do they share common targets? Arthritis Rheumatol. 70, 277–286 (2018).
Bradl, M. et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 66, 630–643 (2009).
Shimizu, F. et al. Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci. Transl. Med. 9, eaai9111 (2017).
Alexopoulos, H. et al. Anti-aquaporin-4 autoantibodies in systemic lupus erythematosus persist for years and induce astrocytic cytotoxicity but not CNS disease. J. Neuroimmunol. 289, 8–11 (2015).
Conti, F. et al. Autoantibody profile in systemic lupus erythematosus with psychiatric manifestations: a role for anti-endothelial-cell antibodies. Arthritis Res. Ther. 6, R366–R372 (2004).
Song, J., Park, Y. B., Lee, W. K., Lee, K. H. & Lee, S. K. Clinical associations of anti-endothelial cell antibodies in patients with systemic lupus erythematosus. Rheumatol. Int. 20, 1–7 (2000).
Nara, H., Okamoto, H., Minota, S. & Yoshio, T. Mouse monoclonal anti-human thrombomodulin antibodies bind to and activate endothelial cells through NF-κB activation in vitro. Arthritis Rheum. 54, 1629–1637 (2006).
Frampton, G. et al. Identification of candidate endothelial cell autoantigens in systemic lupus erythematosus using a molecular cloning strategy: a role for ribosomal P protein P0 as an endothelial cell autoantigen. Rheumatology (Oxford) 39, 1114–1120 (2000).
Williams, R. C. Jr., Sugiura, K. & Tan, E. M. Antibodies to microtubule-associated protein 2 in patients with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 50, 1239–1247 (2004).
Yamada, Y. et al. Antibodies to microtubule-associated protein-2 in the cerebrospinal fluid are a useful diagnostic biomarker for neuropsychiatric systemic lupus erythematosus. Mod. Rheum. 26, 562–568 (2016).
Matsui, T. et al. Identification of novel keratinocyte-secreted peptides dermokine-alpha/-beta and a new stratified epithelium-secreted protein gene complex on human chromosome 19q13.1. Genomics 84, 384–397 (2004).
Park, G. T., Lim, S. E., Jang, S. I. & Morasso, M. I. Suprabasin, a novel epidermal differentiation marker and potential cornified envelope precursor. J. Biol. Chem. 277, 45195–45202 (2002).
Ichinose, K. et al. Novel anti-suprabasin antibodies may contribute to the pathogenesis of neuropsychiatric systemic lupus erythematosus. Clin. Immunol. 193, 123–130 (2018).
James, W. G., Bullard, D. C. & Hickey, M. J. Critical role of the alpha 4 integrin/VCAM-1 pathway in cerebral leukocyte trafficking in lupus-prone MRL/fas (lpr) mice. J. Immunol. 170, 520–527 (2003).
Crispin, J. C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 (2008).
Shivakumar, S., Tsokos, G. C. & Datta, S. K. T cell receptor alpha/beta expressing double-negative (CD4–/CD8–) and CD4+T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J. Immunol. 143, 103–112 (1989).
Jain, S., Stock, A., Macian, F. & Putterman, C. A distinct T follicular helper cell subset infiltrates the brain in murine neuropsychiatric lupus. Front. Immunol. 9, 487 (2018).
Crispin, J. C. et al. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol. Med. 16, 47–57 (2010).
Chalmers, S. A. et al. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J. Autoimmun. 57, 42–52 (2015).
Menke, J. et al. Sunlight triggers cutaneous lupus through a CSF-1-dependent mechanism in MRL-Fas(lpr) mice. J. Immunol. 181, 7367–7379 (2008).
Crupi, R. et al. Reduced adult neurogenesis and altered emotional behaviors in autoimmune-prone B cell activating factor transgenic mice. Biol. Psychiatry 67, 558–566 (2010).
Mondal, T. K., Saha, S. K., Miller, V. M., Seegal, R. F. & Lawrence, D. A. Autoantibody-mediated neuroinflammation: pathogenesis of neuropsychiatric systemic lupus erythematosus in the NZM88 murine model. Brain Behav. Immun. 22, 949–959 (2008).
Chalmers, S. A. et al. Highly selective inhibition of Bruton’s tyrosine kinase attenuates skin and brain disease in murine lupus. Arthritis Res. Ther. 20, 10 (2018).
Hanly, J. G., Walsh, N. M. & Sangalang, V. Brain pathology in systemic lupus erythematosus. J. Rheumatol. 19, 732–741 (1992).
Duprez, T., Nzeusseu, A., Peeters, A. & Houssiau, F. A. Selective involvement of the choroid plexus on cerebral magnetic resonance images: a new radiological sign in patients with systemic lupus erythematosus with neurological symptoms. J. Rheumatol. 28, 387–391 (2001).
Li, Y. et al. Behavioral deficits are accompanied by immunological and neurochemical changes in a mouse model for neuropsychiatric lupus (NP-SLE). Int. J. Mol. Sci. 16, 15150–15171 (2015).
Shiozawa, S., Kuroki, Y., Kim, M., Hirohata, S. & Ogino, T. Interferon-alpha in lupus psychosis. Arthritis Rheum. 35, 417–422 (1992).
Fragoso-Loyo, H., Atisha-Fregoso, Y., Llorente, L. & Sanchez-Guerrero, J. Inflammatory profile in cerebrospinal fluid of patients with headache as a manifestation of neuropsychiatric systemic lupus erythematosus. Rheumatology (Oxford) 52, 2218–2222 (2013).
Santer, D. M., Yoshio, T., Minota, S., Moller, T. & Elkon, K. B. Potent induction of IFN-α and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J. Immunol. 182, 1192–1201 (2009).
Ronnblom, L., Alm, G. V. & Eloranta, M. L. Type I interferon and lupus. Curr. Opin. Rheumatol. 21, 471–477 (2009).
Karageorgas, T. P., Tseronis, D. D. & Mavragani, C. P. Activation of type I interferon pathway in systemic lupus erythematosus: association with distinct clinical phenotypes. J. Biomed. Biotechnol. 2011, 273907 (2011).
Yoshio, T. et al. IL-6, IL-8, IP-10, MCP-1 and G-CSF are significantly increased in cerebrospinal fluid but not in sera of patients with central neuropsychiatric lupus erythematosus. Lupus 25, 997–1003 (2016).
Fragoso-Loyo, H., Atisha-Fregoso, Y., Nunez-Alvarez, C. A., Llorente, L. & Sanchez-Guerrero, J. Utility of interferon-alpha as a biomarker in central neuropsychiatric involvement in systemic lupus erythematosus. J. Rheumatol 39, 504–509 (2012).
Wen, J. et al. Inhibiting TWEAK (TNF-like weak inducer of apoptosis) signaling ameliorates blood brain barrier integrity and neuronal damage in neuropsychiatric lupus prone MRL/lpr mice. J. Immunol. 192, 1 (2014).
Wen, J. et al. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J. Autoimmun. 43, 44–54 (2013).
Fragoso-Loyo, H., Atisha-Fregoso, Y., Nunez-Alvarez, C. A. & Llorente, L. Utility of TWEAK to assess neuropsychiatric disease activity in systemic lupus erythematosus. Lupus 25, 364–369 (2016).
Katsumata, Y. et al. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J. Rheumatol. 34, 2010–2017 (2007).
Hirohata, S. et al. Accuracy of cerebrospinal fluid IL-6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin. Rheumatol. 28, 1319–1323 (2009).
Asano, T. et al. Evaluation of blood-brain barrier function by quotient alpha2 macroglobulin and its relationship with interleukin-6 and complement component 3 levels in neuropsychiatric systemic lupus erythematosus. PLOS ONE 12, e0186414 (2017).
Ichinose, K. et al. Distinguishing the cerebrospinal fluid cytokine profile in neuropsychiatric systemic lupus erythematosus from other autoimmune neurological diseases. Clin. Immunol. 157, 114–120 (2015).
Wang, J. B. et al. Role of IL-1β, IL-6, IL-8 and IFN-γ in pathogenesis of central nervous system neuropsychiatric systemic lupus erythematous. Int. J. Clin. Exp. Med. 8, 16658–16663 (2015).
Plog, B. A. & Nedergaard, M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu. Rev. Pathol. 13, 379–394 (2018).
Gilkeson, G. S. Complement-targeted therapies in lupus. Curr. Treatm Opt. Rheumatol. 1, 10–18 (2015).
Alexander, J. J., Jacob, A., Bao, L., Macdonald, R. L. & Quigg, R. J. Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. J. Immunol. 175, 8312–8319 (2005).
Jongen, P. J., Boerbooms, A. M., Lamers, K. J., Raes, B. C. & Vierwinden, G. Diffuse CNS involvement in systemic lupus erythematosus: intrathecal synthesis of the 4th component of complement. Neurology 40, 1593–1596 (1990).
Jongen, P. J. et al. Cerebrospinal fluid C3 and C4 indexes in immunological disorders of the central nervous system. Acta Neurol. Scand. 101, 116–121 (2000).
Sakuma, Y., Nagai, T., Yoshio, T. & Hirohata, S. Differential activation mechanisms of serum C5a in lupus nephritis and neuropsychiatric systemic lupus erythematosus. Mod. Rheumatol. 27, 292–297 (2017).
Dobrowolski, C. & Erkan, D. Treatment of antiphospholipid syndrome beyond anticoagulation. Clin. Immunol. https://doi.org/10.1016/j.clim.2018.03.001 (2018).
Kronbichler, A. et al. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine 93, e143 (2014).
Shapira, I., Andrade, D., Allen, S. L. & Salmon, J. E. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 64, 2719–2723 (2012).
Paul, F., Murphy, O., Pardo, S. & Levy, M. Investigational drugs in development to prevent neuromyelitis optica relapses. Expert Opin. Investig. Drugs 27, 265–271 (2018).
Lei, H. W. et al. Neuropsychiatric involvement in lupus is associated with the Nogo-a/NgR1 pathway. J. Neuroimmunol. 311, 22–28 (2017).
Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Schrepf, A. et al. A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat. Commun. 9, 2243 (2018).
Nystedt, J. et al. Altered white matter microstructure in lupus patients: a diffusion tensor imaging study. Arthritis Res. Ther. 20, 21 (2018).
Ceccarelli, F. et al. Genetic factors in systemic lupus erythematosus: contribution to disease phenotype. J. Immunol. Res. 2015, 745647 (2015).
de Vries, B. et al. TREX1 gene variant in neuropsychiatric systemic lupus erythematosus. Ann. Rheum. Dis. 69, 1886–1887 (2010).
Namjou, B. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 12, 270–279 (2011).
Lundstrom, E. et al. HLA-DRB1*04/*13 alleles are associated with vascular disease and antiphospholipid antibodies in systemic lupus erythematosus. Ann. Rheum. Dis. 72, 1018–1025 (2013).
Rullo, O. J. & Tsao, B. P. Recent insights into the genetic basis of systemic lupus erythematosus. Ann. Rheum. Dis. 72 (Suppl. 2), ii56–ii61 (2013).
Koga, M. et al. Cumulative association of eight susceptibility genes with systemic lupus erythematosus in a Japanese female population. J. Hum. Gen. 56, 503–507 (2011).
Barile-Fabris, L. et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann. Rheum. Dis. 64, 620–625 (2005).
Mok, C. C., Lau, C. S. & Wong, R. W. Treatment of lupus psychosis with oral cyclophosphamide followed by azathioprine maintenance: an open-label study. Am. J. Med. 115, 59–62 (2003).
Tokunaga, M. et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann. Rheum. Dis. 66, 470–475 (2007).
Dale, R. C. et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 83, 142–150 (2014).
Jacob, A. et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch. Neurol. 65, 1443–1448 (2008).
Pranzatelli, M. R. et al. Rituximab (anti-CD20) adjunctive therapy for opsoclonus-myoclonus syndrome. J. Pediatr. Hematol. Oncol. 28, 585–593 (2006).
Titulaer, M. J. et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 12, 157–165 (2013).
Manzi, S. et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann. Rheum. Dis. 71, 1833–1838 (2012).
Hanly, J. G. in Systemic Lupus Erythematosus 5th edn (ed. Lahita, R. G.) 727–746 (Elsevier, 2005).
Erkan, D., Salmon, J. & Lockshin, M. in Kelley and Firestein’s Textbook of Rheumatology (ed. Firestein, G. S.) 1389–1399 (Elsevier, 2017).
Meroni, P. L. et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-β2-glycoprotein I) antibodies: effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 44, 2870–2878 (2001).
Jung, H. et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 62, 863–868 (2010).
Cervera, R. CAPS Registry. Lupus 21, 755–757 (2012).
Furie, R. et al. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017).
Chitu, V. & Stanley, E. R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 (2006).
Chalmers, S. A. et al. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clin. Immunol. 185, 100–108 (2017).
Hendriks, R. W. Drug discovery: new Btk inhibitor holds promise. Nat. Chem. Biol. 7, 4–5 (2011).
Jongstra-Bilen, J. et al. Dual functions of Bruton’s tyrosine kinase and Tec kinase during Fcγ receptor-induced signaling and phagocytosis. J. Immunol. 181, 288–298 (2008).
Ni Gabhann, J. et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PLOS ONE 9, e85834 (2014).
Chalmers, S. A. et al. Therapeutic blockade of immune complex-mediated glomerulonephritis by highly selective inhibition of Bruton’s tyrosine kinase. Sci. Rep. 6, 26164 (2016).
Mina-Osorio, P. et al. Suppression of glomerulonephritis in lupus-prone NZB × NZW mice by RN486, a selective inhibitor of Bruton’s tyrosine kinase. Arthritis Rheum. 65, 2380–2391 (2013).
Rankin, A. L. et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J. Immunol. 191, 4540–4550 (2013).
Byrd, J. C. et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369, 32–42 (2013).
Zardi, E. M., Taccone, A., Marigliano, B., Margiotta, D. P. & Afeltra, A. Neuropsychiatric systemic lupus erythematosus: tools for the diagnosis. Autoimmun. Rev. 13, 831–839 (2014).
Kivity, S., Agmon-Levin, N., Zandman-Goddard, G., Chapman, J. & Shoenfeld, Y. Neuropsychiatric lupus: a mosaic of clinical presentations. BMC Med. 13, 43 (2015).
Zhang, L., Fu, T., Yin, R., Zhang, Q. & Shen, B. Prevalence of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. BMC Psychiatry 17, 70 (2017).
Torreggiani, S. et al. Chorea, a little-known manifestation in systemic lupus erythematosus: short literature review and four case reports. Pediatr. Rheumatol. 11, 36 (2013).
Li, X. Y., Xiao, H. B. & Pai, P. Myelitis in systemic lupus erythematosus. J. Clin. Neurosci. 44, 18–22 (2017).
Piga, M. et al. Demyelinating syndrome in SLE encompasses different subtypes: do we need new classification criteria? Pooled results from systematic literature review and monocentric cohort analysis. Autoimmun. Rev. 16, 244–252 (2017).
Fragoso-Loyo, H. et al. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum. 56, 1242–1250 (2007).
Dellalibera-Joviliano, R., Dos Reis, M. L., Cunha Fde, Q. & Donadi, E. A. Kinins and cytokines in plasma and cerebrospinal fluid of patients with neuropsychiatric lupus. J. Rheumatol. 30, 485–492 (2003).
Baraczka, K., Nekam, K., Pozsonyi, T., Szuts, I. & Ormos, G. Investigation of cytokine (tumor necrosis factor-alpha, interleukin-6, interleukin-10) concentrations in the cerebrospinal fluid of female patients with multiple sclerosis and systemic lupus erythematosus. Eur. J. Neurol. 11, 37–42 (2004).
Quaresma, M. V. et al. Anti-TNF-α and hydralazine drug-induced lupus. An. Bras. Dermatol. 90, 125–129 (2015).
Groom, J. R. et al. BAFF and MyD88 signals promote a lupus like disease independent of T cells. J. Exp. Med. 204, 1959–1971 (2007).
Zhang, J. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 166, 6–10 (2001).
Crow, M. K. Type I interferon in the pathogenesis of lupus. J. Immunol. 192, 5459–5468 (2014).
Baechler, E. C., Gregersen, P. K. & Behrens, T. W. The emerging role of interferon in human systemic lupus erythematosus. Curr. Opin. Immunol. 16, 801–807 (2004).
Wichers, M. & Maes, M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int. J. Neuropsychopharmacol. 5, 375–388 (2002).
Stock, A. D., Wen, J. & Putterman, C. Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front. Immunol. 4, 484 (2013).
Ronnblom, L. & Elkon, K. B. Cytokines as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 339–347 (2010).
Acknowledgements
N.S. was supported by the Hospital for Special Surgery Research Institute Rheumatology Training Program grant (T32 AR071302). A.D.S. was supported by the Albert Einstein College of Medicine Medical Scientist Training grant (T32-GM007822). C.P. was supported by an R01 grant from the US National Institute of Arthritis and Musculoskeletal Diseases (AR065594).
Reviewer information
Nature Reviews Rheumatology thanks S. Hirohata and the other anonymous reviewers, for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article and contributed to discussions of its content as well as the writing and review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.P. declares that he has received research funding from Biogen Idec for studies of the TNF-like weak inducer of apoptosis (TWEAK) pathway and from Boehringer Ingelheim for studies of tyrosine-protein kinase BTK inhibition in animal models of lupus. N.S. and A.D.S. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schwartz, N., Stock, A.D. & Putterman, C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 15, 137–152 (2019). https://doi.org/10.1038/s41584-018-0156-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0156-8
This article is cited by
-
Constitutive knockout of interleukin-6 ameliorates memory deficits and entorhinal astrocytosis in the MRL/lpr mouse model of neuropsychiatric lupus
Journal of Neuroinflammation (2024)
-
The gateway reflex regulates tissue-specific autoimmune diseases
Inflammation and Regeneration (2024)
-
What is known about the effects of vitamin D in neuropsychiatric lupus?
Advances in Rheumatology (2024)
-
Brain 18 F-FDG PET reveals cortico-subcortical hypermetabolic dysfunction in juvenile neuropsychiatric systemic lupus erythematosus
EJNMMI Research (2024)
-
Interaction of 5-HTTLPR and SLE disease status on resting-state brain function
Arthritis Research & Therapy (2024)