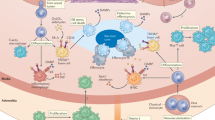
Abstract
Cardiovascular disease is the leading cause of death worldwide, and it commonly results from atherosclerotic plaque progression. One of the increasingly recognized drivers of atherosclerosis is dysfunctional efferocytosis, a homeostatic mechanism responsible for the clearance of dead cells and the resolution of inflammation. In atherosclerosis, the capacity of phagocytes to participate in efferocytosis is hampered, leading to the accumulation of apoptotic and necrotic tissue within the plaque, which results in enlargement of the necrotic core, increased luminal stenosis and plaque inflammation, and predisposition to plaque rupture or erosion. In this Review, we describe the different forms of programmed cell death that can occur in the atherosclerotic plaque and highlight the efferocytic machinery that is normally implicated in cardiovascular physiology. We then discuss the mechanisms by which efferocytosis fails in atherosclerosis and other cardiovascular and cardiometabolic diseases, including myocardial infarction and diabetes mellitus, and discuss therapeutic approaches that might reverse this pathological process.
Key points
-
Efferocytosis is a highly regulated and immunologically silent process through which diseased and dying cells are engulfed by phagocytes for clearance.
-
Integration of multiple signalling pathways involving an array of ligands and receptors ultimately determines whether apoptotic cells are phagocytosed.
-
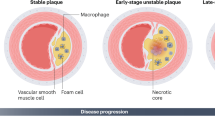
Efferocytosis is impaired in atherosclerotic cardiovascular disease, secondary to inherited genetic variation, necrotic cell death, local inflammation and competitive inhibition by oxidized LDL.
-
The mechanism of cell death influences the capacity of phagocytes to perform efferocytosis.
-
A promising therapeutic strategy for cardiovascular disease is targeting the CD47–SIRPα axis, which might help to attenuate atherosclerosis and cardiac fibrosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Crisby, M. et al. Cell death in human atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis 130, 17–27 (1997).
Neunteufl, T. et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 129, 111–118 (1997).
Bennett, M. R. Apoptosis of vascular smooth muscle cells in vascular remodelling and atherosclerotic plaque rupture. Cardiovasc. Res. 41, 361–368 (1999).
Kockx, M. M. & Herman, A. G. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc. Res. 45, 736–746 (2000).
Wada, Y. et al. In vitro model of atherosclerosis using coculture of arterial wall cells and macrophage. Yonsei Med. J. 41, 740–755 (2000).
Lazar, M. A. Progress in cardiovascular biology: PPAR for the course. Nat. Med. 7, 23–24 (2001).
Bennett, M. R., Evan, G. I. & Schwartz, S. M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Invest. 95, 2266–2274 (1995).
Agewall, S. Anticoagulation, atherosclerosis, and heart failure. Eur. Hear. J. Cardiovasc. Pharmacother. 3, 1–2 (2017).
Bevan, G. H. & Solaru, K. T. W. Evidence-based medical management of peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 40, 541–553 (2020).
Sarmah, D. et al. The role of inflammasomes in atherosclerosis and stroke pathogenesis. Curr. Pharm. Des. 26, 4234–4245 (2020).
Koton, S. et al. Association of ischemic stroke incidence, severity, and recurrence with dementia in the atherosclerosis risk in communities cohort study. JAMA Neurol. 79, 271–280 (2022).
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Vaduganathan, M., Mensah, G. A., Turco, J. V., Fuster, V. & Roth, G. A. The global burden of cardiovascular diseases and risk a compass for future health. J. Am. Coll. Cardiol. 80, 2361–2371 (2022).
Bozkurt, B. et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation 134, e535–e578 (2016).
Dasagrandhi, D., Muthuswamy, A. & Swaminathan, J. K. Atherosclerosis: nexus of vascular dynamics and cellular cross talks. Mol. Cell Biochem. 477, 571–584 (2022).
Huang, C.-L. et al. Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS ONE 9, e90201 (2014).
Liu, J. et al. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 25, 174–179 (2005).
Rochette, L. et al. Interplay between efferocytosis and atherosclerosis. Arch. Cardiovasc. Dis. 116, 474–484 (2023).
Savill, J. Recognition and phagocytosis of cells undergoing apoptosis. Br. Med. Bull. 53, 491–508 (1997).
Henson, P. M., Bratton, D. L. & Fadok, V. A. Apoptotic cell removal. Curr. Biol. 11, R795–R805 (2001).
Morioka, S., Maueröder, C. & Ravichandran, K. S. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50, 1149–1162 (2019).
Lin, D. et al. Efferocytosis and its associated cytokines: a light on non-tumor and tumor diseases? Mol. Ther. - Oncolytics 17, 394–407 (2020).
Thorp, E. B. Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis 15, 1124–1136 (2010).
Gerlach, B. D. et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 27, 525–539 (2020).
Gerlach, B. D. et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 33, 2445–2463.e8 (2021).
Mehrotra, P. & Ravichandran, K. S. Drugging the efferocytosis process: concepts and opportunities. Nat. Rev. Drug. Discov. 21, 601–620 (2022).
Cabrera, J. T. O. & Makino, A. Efferocytosis of vascular cells in cardiovascular disease. Pharmacol. Ther. 229, 107919 (2022).
Wang, Y. et al. Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc. Natl Acad. Sci. USA 117, 15818–15826 (2020).
Sorokin, V. et al. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front. Immunol. 11, 599415 (2020).
Tauber, A. I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 4, 897–901 (2003).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992).
Franc, N. C., Dimarcq, J.-L., Lagueux, M., Hoffmann, J. & Ezekowitz, R. A. B. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 4, 431–443 (1996).
Abrams, J. M., White, K., Fessler, L. I. & Steller, H. Programmed cell death during Drosophila embryogenesis. Development 117, 29–43 (1993).
White, K. et al. Genetic control of programmed cell death in Drosophila. Science 264, 677–683 (1994).
Ellis, R. E., Jacobson, D. M. & Horvitz, H. R. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 129, 79–94 (1991).
Wang, X. & Yang, C. Programmed cell death and clearance of cell corpses in Caenorhabditis elegans. Cell. Mol. Life Sci. 73, 2221–2236 (2016).
Shi, J. et al. A genome-wide CRISPR screen identifies WDFY3 as a regulator of macrophage efferocytosis. Nat. Commun. 13, 7929 (2022).
Kamber, R. A. et al. Inter-cellular CRISPR screens reveal regulators of cancer cell phagocytosis. Nature 597, 549–554 (2021).
Haney, M. S. et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat. Genet. 7, 1716–1727 (2018).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021).
Lin, X. et al. Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis. Arch. Biochem. Biophys. 715, 109098 (2022).
Puylaert, P., Zurek, M., Rayner, K. J., Meyer, G. R. Y. D. & Martinet, W. Regulated necrosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 42, 1283–1306 (2022).
Bertheloot, D., Latz, E. & Franklin, B. S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell. Mol. Immunol. 18, 1106–1121 (2021).
Vasudevan, S. O., Behl, B. & Rathinam, V. A. Pyroptosis-induced inflammation and tissue damage. Semin. Immunol. 69, 101781 (2023).
Berghe, T. V. et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 (2010).
Kojima, Y., Weissman, I. L. & Leeper, N. J. The role of efferocytosis in atherosclerosis. Circulation 135, 476–489 (2017).
Wang, D. et al. The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomed. Pharmacother. 163, 114830 (2023).
Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Robertson, L. E. et al. Induction of apoptotic cell death in chronic lymphocytic leukemia by 2-chloro-2′-deoxyadenosine and 9-β-D-arabinosyl-2-fluoroadenine. Blood 81, 143–150 (1993).
Fadok, V. A. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 (2000).
Bevers, E. M., Comfurius, P., Dekkers, D. W. C. & Zwaal, R. F. A. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta 1439, 317–330 (1999).
Roelofsen, B. Molecular architecture and dynamics of the plasma membrane lipid bilayer: the red blood cell as a model. Infection 19, S206–S209 (1991).
Segawa, K. et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168 (2014).
Souilhol, C. et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat. Rev. Cardiol. 17, 52–63 (2020).
Kaw, K. et al. Smooth muscle α-actin missense variant promotes atherosclerosis through modulation of intracellular cholesterol in smooth muscle cells. Eur. Hear. J. 44, 2713–2726 (2023).
Kattoor, A. J., Pothineni, N. V. K., Palagiri, D. & Mehta, J. L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 19, 42 (2017).
Hartley, A., Haskard, D. & Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis – novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 29, 22–26 (2019).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 73, 22–27 (2019).
Kolodgie, F. D. et al. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am. J. Pathol. 157, 1259–1268 (2000).
Thorp, E. et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9, 474–481 (2009).
Thorp, E. et al. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe−/− mice lacking macrophage Bcl-2. Arterioscler. Thromb. Vasc. Biol. 29, 169–172 (2009).
Arai, S. et al. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 1, 201–213 (2005).
Gautier, E. L. et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation 119, 1795–1804 (2009).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Wu, X. et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 9, 171 (2018).
Fidler, T. P. et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301 (2021).
Park, E. & Chung, S. W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 10, 822 (2019).
Rogers, C. et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 (2017).
Brouckaert, G. et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol. Biol. Cell 15, 1089–1100 (2004).
Crouser, E. D. et al. Monocyte activation by necrotic cells is promoted by mitochondrial proteins and formyl peptide receptors. Crit. Care Med. 37, 2000–2009 (2009).
Freeman, S. A. & Grinstein, S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol. Rev. 262, 193–215 (2014).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Truman, L. A. et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112, 5026–5036 (2008).
Lesnik, P., Haskell, C. A. & Charo, I. F. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J. Clin. Invest. 111, 333–340 (2003).
Narahari, A. K. et al. ATP and large signaling metabolites flux through caspase-activated pannexin 1 channels. eLife 10, e64787 (2021).
Bours, M. J. L., Swennen, E. L. R., Virgilio, F. D., Cronstein, B. N. & Dagnelie, P. C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 (2006).
Junger, W. G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 (2011).
Erb, L. & Weisman, G. A. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1, 789–803 (2012).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Kage, F. et al. Lamellipodia-like actin networks in cells lacking WAVE regulatory complex. J. Cell Sci. 135, jcs260364 (2022).
Kronlage, M. et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 3, ra55 (2010).
Hochhauser, E. et al. P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo. Purinergic Signal. 9, 633–642 (2013).
Chen, X. et al. Endothelial cell-specific deletion of P2Y2 receptor promotes plaque stability in atherosclerosis-susceptible ApoE-null mice. Arterioscler. Thromb. Vasc. Biol. 37, 75–83 (2018).
Weiß, E. & Kretschmer, D. Formyl-peptide receptors in infection, inflammation, and cancer. Trends Immunol. 39, 815–829 (2018).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Yoo, S. K., Starnes, T. W., Deng, Q. & Huttenlocher, A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109–112 (2011).
Nakao, N. et al. Hydrogen peroxide induces the production of tumor necrosis factor-α in RAW 264.7 macrophage cells via activation of p38 and stress-activated protein kinase. Innate Immun. 14, 190–196 (2008).
Chekeni, F. B. et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010).
Elliott, M. R. & Ravichandran, K. S. The dynamics of apoptotic cell clearance. Dev. Cell 38, 147–160 (2016).
Virgilio, F. D. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol. Sci. 28, 465–472 (2007).
Iyer, S. S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl Acad. Sci. USA 106, 20388–20393 (2009).
Liu, M. et al. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE−/− mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol. Sin. 34, 830–836 (2013).
Hirata, Y. et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis 231, 227–233 (2013).
Wahid, A., Chen, W., Wang, X. & Tang, X. High-mobility group Box 1 serves as an inflammation driver of cardiovascular disease. Biomed. Pharmacother. 139, 111555 (2021).
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Fan, H. et al. Association between plasma growth arrest-specific protein 6 and carotid atherosclerosis in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 32, 1917–1923 (2022).
Ait-Oufella, H. et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28, 1429–1431 (2008).
Thorp, E., Cui, D., Schrijvers, D. M., Kuriakose, G. & Tabas, I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 28, 1421–1428 (2008).
Cai, B. et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl Acad. Sci. USA 113, 6526–6531 (2016).
Cai, B. et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J. Clin. Invest. 127, 564–568 (2017).
Tateishi, H. et al. A new inhibitor of ADAM17 composed of a zinc-binding dithiol moiety and a specificity pocket-binding appendage. Chem. Pharm. Bull. 69, 1123–1130 (2021).
Yancey, P. G. et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler. Thromb. Vasc. Biol. 30, 787–795 (2010).
Tcheandjieu, C. et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat. Med. 28, 1679–1692 (2022).
Canuel, M. et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS ONE 8, e64145 (2013).
Liu, S. et al. PCSK9 attenuates efferocytosis in endothelial cells and promotes vascular aging. Theranostics 13, 2914–2929 (2023).
Kojima, Y. et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J. Clin. Invest. 124, 1083–1097 (2014).
Anandan, V. et al. Cyclophilin A impairs efferocytosis and accelerates atherosclerosis by overexpressing CD 47 and down-regulating calreticulin. Cells 10, 3598 (2021).
Aragam, K. G. et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat. Genet. 54, 1803–1815 (2022).
Ait-Oufella, H. et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115, 2168–2177 (2007).
Jarr, K.-U., Kojima, Y., Weissman, I. L. & Leeper, N. J. 2021 Jeffrey M. Hoeg Award lecture: defining the role of efferocytosis in cardiovascular disease: a focus on the CD47 (cluster of differentiation 47) axis. Arterioscler. Thromb. Vasc. Biol. 42, e145–e154 (2022).
Willingham, S. B. et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA 109, 6662–6667 (2012).
Kojima, Y. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016).
Kaur, S., Cicalese, K. V., Banerjee, R. & Roberts, D. D. Preclinical and clinical development of therapeutic antibodies targeting functions of CD47 in the tumor microenvironment. Antib. Ther. 3, 179–192 (2020).
Pietsch, E. C. et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 7, e536 (2017).
Gilead. Gilead statement on discontinuation of phase 3 ENHANCE-3 study in AML (Gilead, 2024).
Daver, N. G. et al. Tolerability and efficacy of the anticluster of differentiation 47 antibody magrolimab combined with azacitidine in patients with previously untreated AML: phase Ib results. J. Clin. Oncol. 41, 4893–4904 (2023).
Jarr, K.-U. et al. Effect of CD47 blockade on vascular inflammation. N. Engl. J. Med. 384, 382–383 (2021).
Jarr, K.-U. et al. 18F-fluorodeoxyglucose-positron emission tomography imaging detects response to therapeutic intervention and plaque vulnerability in a murine model of advanced atherosclerotic disease – brief report. Arterioscler. Thromb. Vasc. Biol. 40, 2821–2828 (2020).
Singla, B. et al. Loss of myeloid cell-specific SIRPα, but not CD47, attenuates inflammation and suppresses atherosclerosis. Cardiovasc. Res. 118, 3097–3111 (2021).
Tsai, R. K. & Discher, D. E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 180, 989–1003 (2008).
Flores, A. M. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 15, 154–161 (2020).
Flores, A. M. et al. Nanoparticle therapy for vascular diseases. Arterioscler. Thromb. Vasc. Biol. 39, 635–646 (2019).
Wang, X. et al. CD24-Siglec axis is an innate immune checkpoint against metaflammation and metabolic disorder. Cell Metab. 34, 1088–1103.e6 (2022).
Brown, S. et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418, 200–203 (2002).
Hsu, Y.-W. et al. Siglec-E retards atherosclerosis by inhibiting CD36-mediated foam cell formation. J. Biomed. Sci. 28, 5 (2021).
Barkal, A. A. et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 19, 76–84 (2018).
Sinthuwiwat, T. et al. A LILRB1 variant with a decreased ability to phosphorylate SHP-1 leads to autoimmune diseases. Sci. Rep. 12, 15420 (2022).
Peng, D. H. et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat. Commun. 11, 4520 (2020).
Colin, S., Chinetti‐Gbaguidi, G. & Staels, B. Macrophage phenotypes in atherosclerosis. Immunol. Rev. 262, 153–166 (2014).
Kumar, D., Pandit, R. & Yurdagul, A. Mechanisms of continual efferocytosis by macrophages and its role in mitigating atherosclerosis. Immunometabolism 5, e00017 (2023).
Xu, W. et al. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood 107, 4930–4937 (2006).
Yurdagul, A. et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 31, 518–533.e10 (2020).
Cohen, P. L. et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196, 135–140 (2002).
Lee, Y. et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF‐κB activation and enhances inflammatory responses in lipopolysaccharide‐induced acute lung injury. J. Leukoc. Biol. 91, 921–932 (2012).
Choi, J.-Y. et al. Upregulation of mer receptor tyrosine kinase signaling attenuated lipopolysaccharide-induced lung inflammation. J. Pharmacol. Exp. Ther. 344, 447–458 (2013).
Choi, J.-Y. et al. Mer signaling increases the abundance of the transcription factor LXR to promote the resolution of acute sterile inflammation. Sci. Signal. 8, ra21 (2015).
Cai, B. et al. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci. Signal. 11, eaar3721 (2018).
Bardin, M. et al. The resolvin D2–GPR18 axis is expressed in human coronary atherosclerosis and transduces atheroprotection in apolipoprotein E deficient mice. Biochem. Pharmacol. 201, 115075 (2022).
Salic, K. et al. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 250, 158–165 (2016).
Hasturk, H. et al. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler. Thromb. Vasc. Biol. 35, 1123–1133 (2018).
Polumuri, S., Perkins, D. J. & Vogel, S. N. cAMP levels regulate macrophage alternative activation marker expression. Innate Immun. 27, 133–142 (2020).
Shan, K. et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J. Cell. Mol. Med. 24, 8045–8056 (2020).
Molaie, M. et al. Imbalanced serum levels of resolvin E1 (RvE1) and leukotriene B4 (LTB4) may contribute to the pathogenesis of atherosclerosis. Prostaglandins Other Lipid Mediat. 169, 106781 (2023).
Zhang, S. et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 29, 443–456.e5 (2019).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2018).
Nissen, S. E. When is a placebo not a placebo. JAMA Cardiol. 7, 1183–1184 (2022).
Daida, H. Randomized trial for evaluating secondary prevention efficacy of combination therapy – statin and eicosapentaenoic acid. Circulation 146, e569–e611 (2022).
Lee, Y.-J. et al. STAT6 signaling mediates PPARγ activation and resolution of acute sterile inflammation in mice. Cells 10, 501 (2021).
Xian, X. et al. LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis. eLife 6, e29292 (2017).
A-Gonzalez, N. et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 (2009).
Ivanov, S. et al. Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Atherosclerosis 287, e77 (2019).
Endo-Umeda, K. et al. Myeloid LXR (liver X receptor) deficiency induces inflammatory gene expression in foamy macrophages and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 42, 719–731 (2022).
Yurdagul, A. Jr et al. ODC (ornithine decarboxylase)-dependent putrescine synthesis maintains MerTK (MER yrosine-protein kinase) expression to drive resolution. Arterioscler. Thromb. Vasc. Biol. 41, e144–e159 (2021).
Cai, W. et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 4, e131355 (2019).
Morioka, S. et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563, 714–718 (2018).
Wang, Y.-T. et al. Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments. Cell Metab. 35, 316–331.e6 (2023).
Schrijvers, D. M., Meyer, G. R. Y.De, Kockx, M. M., Herman, A. G. & Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 1256–1261 (2005).
Yurdagul, A., Doran, A. C., Cai, B., Fredman, G. & Tabas, I. A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 4, 86 (2018).
Silvestre-Roig, C. et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240 (2019).
Kockx, M. M. et al. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 97, 2307–2315 (1998).
Yvan-Charvet, L. et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ. Res. 106, 1861–1869 (2010).
Kannan, K. & Jain, S. K. Oxidative stress and apoptosis. Pathophysiology 7, 153–163 (2000).
Sachet, M., Liang, Y. Y. & Oehler, R. The immune response to secondary necrotic cells. Apoptosis 22, 1189–1204 (2017).
Wright, M. B. et al. Compounds targeting OSBPL7 increase ABCA1-dependent cholesterol efflux preserving kidney function in two models of kidney disease. Nat. Commun. 12, 4662 (2021).
Hoeffner, N., Paul, A. & Goo, Y.-H. Drug screen identifies verteporfin as a regulator of lipid metabolism in macrophage foam cells. Sci. Rep. 13, 19588 (2023).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Sambrano, G. R., Parthasarathy, S. & Steinberg, D. Recognition of oxidatively damaged erythrocytes by a macrophage receptor with specificity for oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 91, 3265–3269 (1994).
Sambrano, G. R. & Steinberg, D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc. Natl Acad. Sci. USA 92, 1396–1400 (1995).
Miller, Y. I. et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278, 1561–1568 (2003).
Wu, R. & Lefvert, A. K. Autoantibodies against oxidized low density lipoproteins (oxLDL): characterization of antibody isotype, subclass, affinity and effect on the macrophage uptake of oxLDL. Clin. Exp. Immunol. 102, 174–180 (1995).
Chang, M.-K. et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl Acad. Sci. USA 96, 6353–6358 (1999).
Fraser, D. A. & Tenner, A. J. Innate immune proteins C1q and mannan-binding lectin enhance clearance of atherogenic lipoproteins by human monocytes and macrophages. J. Immunol. 185, 3932–3939 (2010).
Duus, K. et al. Direct interaction between CD91 and C1q. FEBS J. 277, 3526–3537 (2010).
Pulanco, M. C. et al. Complement protein C1q enhances macrophage foam cell survival and efferocytosis. J. Immunol. 198, 472–480 (2017).
Kojima, Y. et al. Proefferocytic therapy promotes transforming growth factor-β signaling and prevents aneurysm formation. Circulation 137, 750–753 (2018).
Betancur, P. A. et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 8, 14802 (2017).
Zhang, X. et al. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J. Immunother. Cancer 7, 346 (2019).
Bruunsgaard, H., Skinhøj, P., Pedersen, A. N., Schroll, M. & Pedersen, B. K. Ageing, tumour necrosis factor‐alpha (TNF‐α) and atherosclerosis. Clin. Exp. Immunol. 121, 255–260 (2000).
Kohno, K. et al. Inflammatory M1-like macrophages polarized by NK-4 undergo enhanced phenotypic switching to an anti-inflammatory M2-like phenotype upon co-culture with apoptotic cells. J. Inflamm. 18, 2 (2021).
Paul, B. et al. A phase II multi-arm study of magrolimab combinations in patients with relapsed/refractory multiple myeloma. Futur. Oncol. 19, 7–17 (2023).
Sallman, D. A. et al. Magrolimab in combination with azacitidine in patients with higher-risk myelodysplastic syndromes: final results of a phase Ib study. J. Clin. Oncol. 41, 2815–2826 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04827576 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02216409 (2019).
Wong, D. et al. FHL5 controls vascular disease-associated gene programs in smooth muscle cells. Circ. Res. 132, 1144–1161 (2023).
Mosquera, J. V. et al. Integrative single-cell meta-analysis reveals disease-relevant vascular cell states and markers in human atherosclerosis. Cell Rep. 42, 113380 (2023).
Pan, H. et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075 (2020).
Luo, L., Fu, C., Bell, C. F., Wang, Y. & Leeper, N. J. Role of vascular smooth muscle cell clonality in atherosclerosis. Front. Cardiovasc. Med. 10, 1273596 (2023).
Sihombing, M. A. E. M. et al. Unexpected role of nonimmune cells: amateur phagocytes. DNA Cell Biol. 40, 157–171 (2021).
Kirsch, T. et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood 109, 2854–2862 (2007).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015).
Wernig, G. et al. Unifying mechanism for different fibrotic diseases. Proc. Natl Acad. Sci. USA 114, 4757–4762 (2017).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Horckmans, M. et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Hear. J. 38, 187–197 (2017).
Peet, C., Ivetic, A., Bromage, D. I. & Shah, A. M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 116, 1101–1112 (2020).
Dick, S. A. et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 29–39 (2019).
Vagnozzi, R. J. et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577, 405–409 (2020).
Roma-Lavisse, C. et al. M1 and M2 macrophage proteolytic and angiogenic profile analysis in atherosclerotic patients reveals a distinctive profile in type 2 diabetes. Diabetes Vasc. Dis. Res. 12, 279–289 (2015).
DeBerge, M. et al. MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ. Res. 121, 930–940 (2017).
Deng, K.-Q. et al. Restoration of circulating MFGE8 (milk fat globule-EGF factor 8) attenuates cardiac hypertrophy through inhibition of Akt pathway. Hypertension 70, 770–779 (2018).
Zhang, S. et al. Acute CD47 blockade during ischemic myocardial reperfusion enhances phagocytosis-associated cardiac repair. JACC Basic. Transl. Sci. 2, 386–397 (2017).
Mahmoudi, A. et al. Effect of diabetes on efferocytosis process. Mol. Biol. Rep. 49, 10849–10863 (2022).
Maschalidi, S. et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes. Nature 606, 776–784 (2022).
Harris, M. I. Hypercholesterolemia in diabetes and glucose intolerance in the U.S. population. Diabetes Care 14, 366–374 (1991).
Hu, X. et al. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc. Diabetol. 21, 117 (2022).
Hirano, T. Pathophysiology of diabetic dyslipidemia. J. Atheroscler. Thromb. 25, RV17023 (2018).
Okdahl, T. et al. Low-grade inflammation in type 2 diabetes: a cross-sectional study from a Danish diabetes outpatient clinic. BMJ Open. 12, e062188 (2022).
Fiorentino, T., Prioletta, A., Zuo, P. & Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 19, 5695–5703 (2013).
Tseng, H. H. L., Vong, C. T., Kwan, Y. W., Lee, S. M.-Y. & Hoi, M. P. M. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci. Rep. 6, 35016 (2016).
Ye, J. et al. Diabetes mellitus promotes the development of atherosclerosis: the role of NLRP3. Front. Immunol. 13, 900254 (2022).
Crasto, W., Patel, V., Davies, M. J. & Khunti, K. Prevention of microvascular complications of diabetes. Endocrinol. Metab. Clin. North. Am. 50, 431–455 (2021).
Salman, H., Bergman, M., Djaldetti, M. & Bessler, H. Hydrophobic but not hydrophilic statins enhance phagocytosis and decrease apoptosis of human peripheral blood cells in vitro. Biomed. Pharmacother. 62, 41–45 (2008).
Morimoto, K. et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 176, 7657–7665 (2006).
Sahai, E. & Marshall, C. J. RHO-GTPases and cancer. Nat. Rev. Cancer 2, 133–142 (2002).
Tajbakhsh, A. et al. Statin-regulated phagocytosis and efferocytosis in physiological and pathological conditions. Pharmacol. Ther. 238, 108282 (2022).
Jarr, K.-U. et al. The pleiotropic benefits of statins include the ability to reduce CD47 and amplify the effect of pro-efferocytic therapies in atherosclerosis. Nat. Cardiovasc. Res. 1, 253–262 (2022).
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004); correction 354, 778 (2006).
Chan, A. W. et al. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation 105, 691–696 (2002).
Ray, K. K. & Cannon, C. P. Time to benefit: an emerging concept for assessing the efficacy of statin therapy in cardiovascular disease. Crit. Pathw. Cardiol. 4, 43–45 (2005).
McInnes, G., Yee, S. W., Pershad, Y. & Altman, R. B. Genomewide association studies in pharmacogenomics. Clin. Pharmacol. Ther. 110, 637–648 (2021).
Motsinger-Reif, A. A. et al. Genome-wide association studies in pharmacogenomics. Pharmacogenet. Genom. 23, 383–394 (2013).
Nanda, V. et al. CDKN2B regulates TGFβ signaling and smooth muscle cell investment of hypoxic neovessels. Circ. Res. 118, 230–240 (2016).
Kasikara, C. et al. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J. Clin. Invest. 131, e145275 (2021).
Alsheikh, A. J. et al. The landscape of GWAS validation; systematic review identifying 309 validated non-coding variants across 130 human diseases. BMC Med. Genom. 15, 74 (2022).
Feehley, T., O’Donnell, C. W., Mendlein, J., Karande, M. & McCauley, T. Drugging the epigenome in the age of precision medicine. Clin. Epigenetics 15, 6 (2023).
Cappelluti, M. A. et al. Durable and efficient gene silencing in vivo by hit-and-run epigenome editing. Nature 627, 416–423 (2024).
Klann, T. S. et al. CRISPR–Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nat. Biotechnol. 35, 561–568 (2017).
Wennogle, L. P. et al. Comparison of recombinant cyclooxygenase‐2 to native isoforms: aspirin labeling of the active site. FEBS Lett. 371, 315–320 (1995).
Yoo, S. G. K. et al. Aspirin for secondary prevention of cardiovascular disease in 51 low-, middle-, and high-income countries. JAMA 330, 715–724 (2023).
Minikel, E. V. et al. Evaluating drug targets through human loss-of-function genetic variation. Nature 581, 459–464 (2020).
Morioka, S. et al. Chimeric efferocytic receptors improve apoptotic cell clearance and alleviate inflammation. Cell 185, 4887–4903.e17 (2022).
Lafont, A. Basic aspects of plaque vulnerability. Heart 89, 1262 (2003).
McDermott, D. H. et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J. Clin. Invest. 111, 1241–1250 (2003).
Friggeri, A. et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. Am. J. Physiol. Cell Physiol. 299, C1267–C1276 (2010).
Chen, W. et al. The ABCA1-efferocytosis axis: a new strategy to protect against atherosclerosis. Clin. Chim. Acta 518, 1–8 (2021).
Rong, J. et al. Lysophasphatidylcholine stimulates monocyte chemoattractant protein-1 gene expression in rat aortic smooth muscle cells. Asterioscler. Thromb. Vasc. Biol. 22, 1617–1623 (2008).
Gude, D. R. et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine‐1‐phosphate as a “come‐and‐get‐me” signal. FASEB J. 22, 2629–2638 (2008).
Galvani, S. et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 8, ra79 (2015).
Bhatia, V. K. et al. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am. J. Pathol. 170, 416–426 (2007).
Kourtzelis, I. et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 20, 40–49 (2019).
Kakino, A., Fujita, Y., Nakano, A., Horiuchi, S. & Sawamura, T. Developmental endothelial locus-1 (Del-1) inhibits oxidized low-density lipoprotein activity by direct binding, and its overexpression attenuates atherogenesis in mice. Circ. J. 80, 2541–2549 (2016).
van der Meer, J. H., van der Poll, T. & van ‘t Veer, C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood 123, 2460–2469 (2014).
Lutgens, E. et al. Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J. Pathol. 216, 55–63 (2008).
Robins, R. S. et al. Vascular Gas6 contributes to thrombogenesis and promotes tissue factor up-regulation after vessel injury in mice. Blood 121, 692–699 (2013).
Tang, Y. et al. Mertk deficiency affects macrophage directional migration via disruption of cytoskeletal organization. PLoS ONE 10, e0117787 (2015).
Park, D., Hochreiter-Hufford, A. & Ravichandran, K. S. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 19, 346–351 (2009).
Moon, B. et al. Mertk interacts with Tim-4 to enhance Tim-4-mediated efferocytosis. Cells 9, 1625 (2020).
Foks, A. C. et al. Blockade of Tim-1 and Tim-4 enhances atherosclerosis in low-density lipoprotein receptor–deficient mice. Arterioscler. Thromb. Vasc. Biol. 36, 456–465 (2018).
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 (2007).
Fond, A. M., Lee, C. S., Schulman, I. G., Kiss, R. S. & Ravichandran, K. S. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J. Clin. Invest. 125, 2748–2758 (2015).
Park, S.-Y. et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201 (2008).
Kayashima, Y. et al. Reduction of stabilin-2 contributes to a protection against atherosclerosis. Front. Cardiovasc. Med. 9, 818662 (2022).
Jenkins, W. S. et al. In vivo alpha-V beta-3 integrin expression in human aortic atherosclerosis. Heart 105, 1868–1875 (2019).
Tao, H. et al. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J. Lipid Res. 56, 1449–1460 (2015).
Falasca, L. et al. Transglutaminase type II is a key element in the regulation of the anti-inflammatory response elicited by apoptotic cell engulfment. J. Immunol. 174, 7330–7340 (2005).
Thorp, E., Subramanian, M. & Tabas, I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur. J. Immunol. 41, 2515–2518 (2011).
Boisvert, W. A. et al. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler. Thromb. Vasc. Biol. 26, 563–569 (2006).
Isner, J. M., Kearney, M., Bortman, S. & Passeri, J. Apoptosis in human atherosclerosis and restenosis. Circulation 91, 2703–2711 (1995).
Henderson, N. C., Rieder, F. & Wynn, T. A. Fibrosis: from mechanisms to medicines. Nature 587, 555–566 (2020).
Morimoto, K., Janssen, W. J. & Terada, M. Defective efferocytosis by alveolar macrophages in IPF patients. Respir. Med. 106, 1800–1803 (2012).
Shi, H. et al. CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis. Sci. Transl. Med. 14, eabp8309 (2022).
Li, Y. et al. CD47 antibody suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Am. J. Transl. Res. 12, 5908–5923 (2019).
Acknowledgements
N.J.L. is supported by the grants NIH R35HL 144475 and AHA EIA34770065, the Leducq Foundation, the Falk Foundation, and the Greathouse Family Foundation. S.S.A. is supported by the grant NIH T32HL 98049-13.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
N.J.L. is co-founder and director of Bitterroot Bio, a biotechnology company focused on immunomodulatory therapies for cardiovascular disease. S.S.A. declares no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Amanda C. Doran, MacRae F. Linton, and Manikandan Subramanian for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adkar, S.S., Leeper, N.J. Efferocytosis in atherosclerosis. Nat Rev Cardiol (2024). https://doi.org/10.1038/s41569-024-01037-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-024-01037-7