Abstract
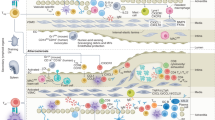
Atherosclerosis is the root cause of many cardiovascular diseases. Extensive research in preclinical models and emerging evidence in humans have established the crucial roles of the innate and adaptive immune systems in driving atherosclerosis-associated chronic inflammation in arterial blood vessels. New techniques have highlighted the enormous heterogeneity of leukocyte subsets in the arterial wall that have pro-inflammatory or regulatory roles in atherogenesis. Understanding the homing and activation pathways of these immune cells, their disease-associated dynamics and their regulation by microbial and metabolic factors will be crucial for the development of clinical interventions for atherosclerosis, including potentially vaccination-based therapeutic strategies. Here, we review key molecular mechanisms of immune cell activation implicated in modulating atherogenesis and provide an update on the contributions of innate and adaptive immune cell subsets in atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Michos, E. D., McEvoy, J. W. & Blumenthal, R. S. Lipid management for the prevention of atherosclerotic cardiovascular disease. N. Engl. J. Med. 381, 1557–1567 (2019).
Sampson, U. K., Fazio, S. & Linton, M. F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 14, 1–10 (2012).
Ridker, P. M. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur. Heart J. 37, 1720–1722 (2016).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Basatemur, G. L., Jorgensen, H. F., Clarke, M. C. H., Bennett, M. R. & Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 16, 727–744 (2019).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Zernecke, A. et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ. Res. 127, 402–426 (2020). This meta-analysis of leukocyte populations in mouse atherosclerotic aortas comprehensively summarizes the transcript profiles and gene signatures of aortic immune cell subsets obtained from nine scRNA-seq studies and two mass cytometry studies.
Pan, H. et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075 (2020).
Wirka, R. C. et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 25, 1280–1289 (2019).
Feil, S. et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 115, 662–667 (2014).
Rayner, K. J. Cell death in the vessel wall: the good, the bad, the ugly. Arterioscler. Thromb. Vasc. Biol. 37, e75–e81 (2017).
Cardoso, L. & Weinbaum, S. Microcalcifications, their genesis, growth, and biomechanical stability in fibrous cap rupture. Adv. Exp. Med. Biol. 1097, 129–155 (2018).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019). This study undertakes an integrated single-cell proteomic and transcriptomic analysis of human atherosclerotic plaques and identifies unique features of plaque T cells and macrophages that are associated with clinical cardiovascular disease.
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127, 1437–1455 (2020).
Back, M., Yurdagul, A. Jr, Tabas, I., Oorni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16, 389–406 (2019).
Spitz, C. et al. Regulatory T cells in atherosclerosis: critical immune regulatory function and therapeutic potential. Cell. Mol. Life Sci. 73, 901–922 (2016).
Tse, K. et al. Atheroprotective vaccination with MHC-II restricted peptides from ApoB-100. Front. Immunol. 4, 493 (2013).
Roy, P., Ali, A. J., Kobiyama, K., Ghosheh, Y. & Ley, K. Opportunities for an atherosclerosis vaccine: from mice to humans. Vaccine 38, 4495–4506 (2020).
Libby, P., Lichtman, A. H. & Hansson, G. K. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 38, 1092–1104 (2013).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Kim, K. W., Ivanov, S. & Williams, J. W. Monocyte recruitment, specification, and function in atherosclerosis. Cells 10, 15 (2020).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007). This study shows that an atherogenic diet induces systemic monocytosis in atherosclerotic mice and it defines the contributions of different chemokine receptors in mediating recruitment of distinct monocyte subsets to atherosclerotic lesions.
Combadiere, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Tabas, I. & Lichtman, A. H. Monocyte-macrophages and T cells in atherosclerosis. Immunity 47, 621–634 (2017).
Jakubzick, C. V., Randolph, G. J. & Henson, P. M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 17, 349–362 (2017).
Marcovecchio, P. M. et al. Scavenger receptor CD36 directs nonclassical monocyte patrolling along the endothelium during early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 37, 2043–2052 (2017).
Quintar, A. et al. Endothelial protective monocyte patrolling in large arteries intensified by Western diet and atherosclerosis. Circ. Res. 120, 1789–1799 (2017).
Narasimhan, P. B., Marcovecchio, P., Hamers, A. A. J. & Hedrick, C. C. Nonclassical monocytes in health and disease. Annu. Rev. Immunol. 37, 439–456 (2019).
Hamers, A. A. J. et al. Human monocyte heterogeneity as revealed by high-dimensional mass cytometry. Arterioscler. Thromb. Vasc. Biol. 39, 25–36 (2019). This study uses high-dimensional single-cell mass cytometry to establish the heterogeneity of human blood monocyte subsets, and it functionally characterizes non-classical monocytes expressing the carbohydrate marker 6-sulfo LacNAc, the frequency of which correlates positively with the severity of coronary artery disease.
Kim, K. et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ. Res. 123, 1127–1142 (2018).
Spann, N. J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012). This study combines lipidomic and transcriptomic analyses to show that macrophage-derived foam cells are associated with suppression, rather than activation, of inflammatory genes and that most of the changes in gene expression and lipid metabolism are triggered by the accumulation of desmosterol, an intermediate in the cholesterol biosynthesis pathway.
Koelwyn, G. J., Corr, E. M., Erbay, E. & Moore, K. J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 19, 526–537 (2018).
Kojima, Y., Weissman, I. L. & Leeper, N. J. The role of efferocytosis in atherosclerosis. Circulation 135, 476–489 (2017).
Moore, K. J. et al. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD Series (part 2). J. Am. Coll. Cardiol. 72, 2181–2197 (2018).
McArdle, S. et al. Migratory and dancing macrophage subsets in atherosclerotic lesions. Circ. Res. 125, 1038–1051 (2019).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
van Gils, J. M. et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 13, 136–143 (2012). This study defines the role of netrin 1, a neuroimmune guidance factor, in promoting atherosclerotic plaque progression through the retention of macrophages within inflamed blood vessels.
Wanschel, A. et al. Neuroimmune guidance cue semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler. Thromb. Vasc. Biol. 33, 886–893 (2013).
Trogan, E. et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl Acad. Sci. USA 103, 3781–3786 (2006).
Feig, J. E. et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998 (2011).
Feig, J. E. et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 (2010).
Potteaux, S. et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Invest. 121, 2025–2036 (2011).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Cochain, C. et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ. Res. 122, 1661–1674 (2018).
Winkels, H. et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ. Res. 122, 1675–1688 (2018).
King, K. R. et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23, 1481–1487 (2017).
Lin, J. D. et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4, e124574 (2019).
Williams, J. W. et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat. Immunol. 21, 1194–1204 (2020). This study characterizes a monocyte-derived arterial resident macrophage population, known as MacAIR, and describes their origins and functions during early and advanced stages of atherosclerosis.
Ensan, S. et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168 (2016). This study uses multiple fate-mapping approaches to show that tissue-resident macrophages in the arterial adventitia arise from embryonic precursor cells and undergo local proliferation.
Lim, H. Y. et al. Hyaluronan receptor LYVE-1expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 49, 326–341.e7 (2018).
Hill, C. A., Fernandez, D. M. & Giannarelli, C. Single cell analyses to understand the immune continuum in atherosclerosis. Atherosclerosis 330, 85–94 (2021).
Zernecke, A. Dendritic cells in atherosclerosis: evidence in mice and humans. Arterioscler. Thromb. Vasc. Biol. 35, 763–770 (2015).
Choi, J. H. et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35, 819–831 (2011).
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
Weber, C. et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Invest. 121, 2898–2910 (2011).
Koltsova, E. K. et al. Dynamic T cell-APC interactions sustain chronic inflammation in. atherosclerosis. J. Clin. Invest. 122, 3114–3126 (2012). This study uses live-cell imaging of explanted mouse atherosclerotic aortas to show antigen-dependent interactions between aortic APCs and CD4+ T cells and that CD4+ T cells from atherosclerotic mice, but not control mice, interact with APCs in the vessel walls of atherosclerotic aorta, suggesting a recall response to an endogenous antigen.
Choi, J. H. et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 206, 497–505 (2009).
Haddad, Y. et al. The dendritic cell receptor DNGR-1 promotes the development of atherosclerosis in mice. Circ. Res. 121, 234–243 (2017).
Clement, M. et al. Deletion of IRF8 (interferon regulatory factor 8)-dependent dendritic cells abrogates proatherogenic adaptive immunity. Circ. Res. 122, 813–820 (2018).
Subramanian, M., Thorp, E., Hansson, G. K. & Tabas, I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. 123, 179–188 (2013). This study shows that MYD88-dependent TLR signalling in CD11c+ DCs, which is important for DC maturation, is necessary for the infiltration of both effector T cells and Treg cells in atherosclerotic lesions but largely has an atheroprotective role through the inhibition of inflammatory monocyte recruitment by Treg cells.
Clement, M. et al. Impaired autophagy in CD11b+ dendritic cells expands CD4+ regulatory T cells and limits atherosclerosis in mice. Circ. Res. 125, 1019–1034 (2019).
Doring, Y. et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125, 1673–1683 (2012).
Sage, A. P. et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 130, 1363–1373 (2014). This study identifies that the pro-atherogenic functions of pDCs are mediated by MHC class II-restricted antigen presentation to CD4+ T cells and that this is independent of IFNα production.
Daissormont, I. T. et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ. Res. 109, 1387–1395 (2011).
Yun, T. J. et al. Indoleamine 2,3-dioxygenase-expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell Metab. 23, 852–866 (2016).
Sage, A. P., Tsiantoulas, D., Binder, C. J. & Mallat, Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 16, 180–196 (2019).
Galkina, E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 (2006).
Butcher, M. J., Wu, C. I., Waseem, T. & Galkina, E. V. CXCR6 regulates the recruitment of pro-inflammatory IL-17A-producing T cells into atherosclerotic aortas. Int. Immunol. 28, 255–261 (2016).
Li, J. et al. CCR5+T-bet+FoxP3+ effector CD4 T cells drive atherosclerosis. Circ. Res. 118, 1540–1552 (2016).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9, 61–67 (2003).
Gencer, S., Evans, B. R., van der Vorst, E. P. C., Doring, Y. & Weber, C. Inflammatory chemokines in atherosclerosis. Cells 10, 226 (2021).
Saigusa, R., Winkels, H. & Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17, 387–401 (2020).
Ley, K., Gerdes, N. & Winkels, H. ATVB distinguished scientist award: how costimulatory and coinhibitory pathways shape atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, 764–777 (2017).
Wolf, D. & Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 124, 315–327 (2019).
Ait-Oufella, H., Taleb, S., Mallat, Z. & Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 969–979 (2011).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Maganto-Garcia, E., Tarrio, M. L., Grabie, N., Bu, D. X. & Lichtman, A. H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 124, 185–195 (2011).
Klingenberg, R. et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 123, 1323–1334 (2013).
Mullick, A. E. et al. Antisense oligonucleotide reduction of apoB-ameliorated atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 52, 885–896 (2011).
Chereshnev, I. et al. Mouse model of heterotopic aortic arch transplantation. J. Surg. Res. 111, 171–176 (2003).
Sharma, M. et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ. Res. 127, 335–353 (2020).
Engelbertsen, D. et al. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler. Thromb. Vasc. Biol. 33, 637–644 (2013).
Engelbertsen, D. et al. Induction of T helper 2 responses against human apolipoprotein B100 does not affect atherosclerosis in ApoE−/− mice. Cardiovasc. Res. 103, 304–312 (2014).
Binder, C. J. et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 (2004).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Knutsson, A. et al. Associations of interleukin-5 with plaque development and cardiovascular events. JACC Basic Transl. Sci. 4, 891–902 (2019).
Cardilo-Reis, L. et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4, 1072–1086 (2012).
Newland, S. A. et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat. Commun. 8, 15781 (2017). This study uses atherosclerotic mouse models that were deficient in group 2 innate lymphoid cells to prove that IL-5 and IL-13 production by innate lymphoid cells has an atheroprotective role and to highlight the functional overlap between innate lymphoid cells and TH cell subsets in modulating atherosclerosis.
Gao, Q. et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 185, 5820–5827 (2010).
Nordlohne, J. et al. Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor a and are normalized by inhibition of interleukin-17A. JACC Basic Transl. Sci. 3, 54–66 (2018).
Smith, E. et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121, 1746–1755 (2010).
Danzaki, K. et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 273–280 (2012).
Gistera, A. et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl. Med. 5, 196ra100 (2013).
Brauner, S. et al. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc. Res. 114, 158–167 (2018).
Gensous, N. et al. T follicular helper cells in autoimmune disorders. Front. Immunol. 9, 1637 (2018).
Ryu, H. et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol. 19, 583–593 (2018).
Clement, M. et al. Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 131, 560–570 (2015).
Nus, M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 23, 601–610 (2017).
Butcher, M. J. et al. Atherosclerosis-driven Treg plasticity results in formation of a dysfunctional subset of plastic IFNγ+ Th1/Tregs. Circ. Res. 119, 1190–1203 (2016). This study shows that inflammatory conditions associated with atherosclerosis can trigger the plasticity of Treg cells and result in the formation of IFNγ-expressing T cells with a mixed TH1 cell and Treg cell phenotype, which have impaired ability to suppress effector T cell proliferation.
Gaddis, D. E. et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 (2018).
Ali, A. J., Makings, J. & Ley, K. Regulatory T cell stability and plasticity in atherosclerosis. Cells 9, 2665 (2020).
Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Srikakulapu, P. et al. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis B-cell responses in aged ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol. 36, 1174–1185 (2016).
Marchini, T., Hansen, S. & Wolf, D. ApoB-specific CD4+ T cells in mouse and human atherosclerosis. Cells 10, 446 (2021).
Kimura, T. et al. Regulatory CD4+ T cells recognize MHC-II-restricted peptide epitopes of apolipoprotein B. Circulation 138, 1130–1143 (2018). This is the first study to detect and characterize APOB-specific autoreactive CD4+ T cells in humans using peptide-specific tetramers and to show the prophylactic potential of human APOB-derived peptide vaccines in mouse models of atherosclerosis.
Wolf, D. et al. Pathogenic autoimmunity in atherosclerosis evolves from initially protective apolipoprotein B100-reactive CD4+ T-regulatory cells. Circulation 142, 1279–1293 (2020). This study uses a novel MHC class II tetramer to undertake detailed phenotypic and transcriptomic analyses of APOB-specific mouse CD4+ T cells and establishes the feasibility of using restimulation assays to detect cytokine-expressing, MHC class II-restricted, antigen-specific T cells in human blood.
Zimmer, S., Grebe, A. & Latz, E. Danger signaling in atherosclerosis. Circ. Res. 116, 323–340 (2015).
Ley, K., Pramod, A. B., Croft, M., Ravichandran, K. S. & Ting, J. P. How mouse macrophages sense what is going on. Front. Immunol. 7, 204 (2016).
Libby, P. Targeting inflammatory pathways in cardiovascular disease: the inflammasome, interleukin-1, interleukin-6 and beyond. Cells 10, 951 (2021).
Miller, Y. I., Choi, S. H., Wiesner, P. & Bae, Y. S. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br. J. Pharmacol. 167, 990–999 (2012).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Grebe, A., Hoss, F. & Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 122, 1722–1740 (2018).
Campbell, L. A. & Rosenfeld, M. E. Infection and atherosclerosis development. Arch. Med. Res. 46, 339–350 (2015).
Pothineni, N. V. K. et al. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J. 38, 3195–3201 (2017).
Wick, G., Jakic, B., Buszko, M., Wick, M. C. & Grundtman, C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 11, 516–529 (2014).
Kearns, A. C. et al. Caspase-1 activation is related with HIV-associated atherosclerosis in an HIV transgenic mouse model and HIV patient cohort. Arterioscler. Thromb. Vasc. Biol. 39, 1762–1775 (2019).
Epstein, S. E., Zhu, J., Najafi, A. H. & Burnett, M. S. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation 119, 3133–3141 (2009).
Andraws, R., Berger, J. S. & Brown, D. L. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA 293, 2641–2647 (2005).
Tang, W. H. W., Backhed, F., Landmesser, U. & Hazen, S. L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 2089–2105 (2019).
Piya, M. K., Harte, A. L. & McTernan, P. G. Metabolic endotoxaemia: is it more than just a gut feeling? Curr. Opin. Lipidol. 24, 78–85 (2013).
Romano, K. A., Vivas, E. I., Amador-Noguez, D. & Rey, F. E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6, e02481 (2015). This study uses ultra-high-pressure liquid chromatography and tandem mass spectrometry to identify choline-consuming, trimethylamine-producing bacterial species in human intestinal isolates and characterizes their relevance in gnotobiotic mice.
Chen, M. L. et al. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart Assoc. 6, e006347 (2017).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013). This study establishes a mechanistic connection between the dietary component l-carnitine, gut microbial metabolism and TMAO-mediated inhibition of cholesterol reverse transport and increased cardiovascular disease risk.
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Zhu, W. et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124 (2016).
Li, X. S. et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 38, 814–824 (2017).
Schiattarella, G. G. et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur. Heart J. 38, 2948–2956 (2017).
Tang, W. H. et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64, 1908–1914 (2014).
Roberts, A. B. et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 24, 1407–1417 (2018).
Wang, Z. et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163, 1585–1595 (2015).
Aldana-Hernandez, P. et al. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr−/− and Apoe−/− male mice. J. Nutr. 150, 249–255 (2020).
Collins, H. L. et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis 244, 29–37 (2016).
Zeisel, S. H. & Warrier, M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 37, 157–181 (2017).
Manor, O. et al. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 24, 935–946 (2018).
Kasahara, K. et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 3, 1461–1471 (2018).
Fatkhullina, A. R. et al. An interleukin-23-interleukin-22 axis regulates intestinal microbial homeostasis to protect from diet-induced atherosclerosis. Immunity 49, 943–957.e9 (2018).
Kurilenko, N., Fatkhullina, A. R., Mazitova, A. & Koltsova, E. K. Act locally, act globally-microbiota, barriers, and cytokines in atherosclerosis. Cells 10, 348 (2021).
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Que, X. et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018). This study generates a transgenic mouse model expressing antibodies to oxLDL to evaluate the pro-atherogenic role of oxidized phospholipids in vivo; it proposes antibody-mediated blockade of oxidized phospholipids as a potential therapeutic strategy for atherosclerosis.
Moore, K. J. & Freeman, M. W. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 26, 1702–1711 (2006).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Menu, P. et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2, e137 (2011).
van der Heijden, T. et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice — brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 (2017).
Weisshaar, S. & Zeitlinger, M. Vaccines targeting PCSK9: a promising alternative to passive immunization with monoclonal antibodies in the management of hyperlipidaemia? Drugs 78, 799–808 (2018).
Nettersheim, F. S., De Vore, L. & Winkels, H. Vaccination in atherosclerosis. Cells 9, 2560 (2020).
Gistera, A. et al. Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis. J. Intern. Med. 281, 383–397 (2017).
Ait-Oufella, H., Lavillegrand, J. R. & Tedgui, A. Regulatory T cell-enhancing therapies to treat atherosclerosis. Cells 10, 723 (2021).
Mauersberger, C., Schunkert, H. & Sager, H. B. Inflammation-related risk loci in genome-wide association studies of coronary artery disease. Cells 10, 440 (2021).
Cole, J. E. et al. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc. Res. 114, 1360–1371 (2018).
Mulligan-Kehoe, M. J. & Simons, M. Vasa vasorum in normal and diseased arteries. Circulation 129, 2557–2566 (2014).
Rademakers, T. et al. Plaque-associated vasa vasorum in aged apolipoprotein E-deficient mice exhibit proatherogenic functional features in vivo. Arterioscler. Thromb. Vasc. Biol. 33, 249–256 (2013).
Kocher, O. & Krieger, M. Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr. Opin. Lipidol. 20, 236–241 (2009).
Pal, R. et al. Carboxy-terminal deletion of the HDL receptor reduces receptor levels in liver and steroidogenic tissues, induces hypercholesterolemia, and causes fatal heart disease. Am. J. Physiol. Heart Circ. Physiol. 311, H1392–H1408 (2016).
Yin, W. et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J. Lipid Res. 53, 51–65 (2012).
Barrett-Connor, E. Menopause, atherosclerosis, and coronary artery disease. Curr. Opin. Pharmacol. 13, 186–191 (2013).
Maeda, N. et al. Anatomical differences and atherosclerosis in apolipoprotein E-deficient mice with 129/SvEv and C57BL/6 genetic backgrounds. Atherosclerosis 195, 75–82 (2007).
Buscher, K. et al. Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat. Commun. 8, 16041 (2017).
Bennett, B. J. et al. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genet. 11, e1005711 (2015).
Mailer, R. K. W., Gistera, A., Polyzos, K. A., Ketelhuth, D. F. J. & Hansson, G. K. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci. Rep. 7, 15655 (2017).
Pollock, A. H. et al. Prolonged intake of dietary lipids alters membrane structure and t cell responses in LDLr−/− mice. J. Immunol. 196, 3993–4002 (2016).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Barrington, W. T. & Lusis, A. J. Atherosclerosis: association between the gut microbiome and atherosclerosis. Nat. Rev. Cardiol. 14, 699–700 (2017).
He, S. et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119 (2019).
Bonaccorsi, I. et al. Symptomatic carotid atherosclerotic plaques are associated with increased infiltration of natural killer (NK) cells and higher serum levels of NK activating receptor ligands. Front. Immunol. 10, 1503 (2019).
Selathurai, A. et al. Natural killer (NK) cells augment atherosclerosis by cytotoxic-dependent mechanisms. Cardiovasc. Res. 102, 128–137 (2014).
Nour-Eldine, W. et al. Genetic depletion or hyperresponsiveness of natural killer cells do not affect atherosclerosis development. Circ. Res. 122, 47–57 (2018).
Kyaw, T. et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 127, 1028–1039 (2013).
Seijkens, T. T. P. et al. Deficiency of the T cell regulator casitas B-cell lymphoma-B aggravates atherosclerosis by inducing CD8+ T cell-mediated macrophage death. Eur. Heart J. 40, 372–382 (2019).
Cochain, C. et al. CD8+ T cells regulate monopoiesis and circulating Ly6C-high monocyte levels in atherosclerosis in mice. Circ. Res. 117, 244–253 (2015).
Dimayuga, P. C. et al. Identification of apoB-100 peptide-specific CD8+ T cells in atherosclerosis. J. Am. Heart Assoc. 6, e005318 (2017).
Schafer, S. & Zernecke, A. CD8+ T cells in atherosclerosis. Cells 10, 37 (2020).
Getz, G. S. & Reardon, C. A. Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol. 14, 304–314 (2017).
Li, Y. et al. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circ. Res. 116, 245–254 (2015).
Vu, D. M. et al. γδT cells are prevalent in the proximal aorta and drive nascent atherosclerotic lesion progression and neutrophilia in hypercholesterolemic mice. PLoS ONE 9, e109416 (2014).
Cheng, H. Y., Wu, R. & Hedrick, C. C. Gammadelta (γδ) T lymphocytes do not impact the development of early atherosclerosis. Atherosclerosis 234, 265–269 (2014).
Poller, W. C., Nahrendorf, M. & Swirski, F. K. Hematopoiesis and cardiovascular disease. Circ. Res. 126, 1061–1085 (2020).
Yvan-Charvet, L. et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010).
Murphy, A. J. et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121, 4138–4149 (2011).
Seijkens, T. et al. Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. FASEB J. 28, 2202–2213 (2014).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012). This study shows that monocyte infiltrates in atherosclerotic lesions do not arise exclusively from bone marrow haematopoietic progenitors but also are produced by haematopoiesis at extramedullary sites, particularly the spleen.
Westerterp, M. et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11, 195–206 (2012).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
McAlpine, C. S. et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Nahrendorf, M. & Swirski, F. K. Lifestyle effects on hematopoiesis and atherosclerosis. Circ. Res. 116, 884–894 (2015).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
Ketelhuth, D. F. J. et al. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc. Res. 115, 1385–1392 (2019).
Perez-Medina, C., Fayad, Z. A. & Mulder, W. J. M. Atherosclerosis immunoimaging by positron emission tomography. Arterioscler. Thromb. Vasc. Biol. 40, 865–873 (2020).
Ouimet, M. et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Invest. 125, 4334–4348 (2015).
Gerriets, V. A. et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 46, 1970–1983 (2016).
Taleb, S. et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 2691–2698 (2007).
Shirai, T. et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213, 337–354 (2016). This study identifies the glycolytic enzyme pyruvate kinase M2 as a crucial molecular link between altered metabolic adaptations and increased inflammation and oxidative stress in monocytes and macrophages from patients with coronary artery disease.
Tomas, L. et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur. Heart J. 39, 2301–2310 (2018). This study conducts targeted metabolic profiling of human carotid plaques and identifies distinct metabolic signatures that distinguish high-risk atherosclerotic plaques from low-risk atherosclerotic plaques.
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175.e14 (2018). This study shows that a Western-style diet high in fat and cholesterol triggers NLRP3-dependent inflammasome activation and long-term epigenetic and metabolic reprogramming of myeloid progenitor cells.
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146.e9 (2018). This study identifies mevalonate, a metabolic intermediate in the cholesterol synthesis pathway, as an inducer of trained immunity in human monocytes.
Flores-Gomez, D., Bekkering, S., Netea, M. G. & Riksen, N. P. Trained immunity in atherosclerotic cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 41, 62–69 (2021).
Baardman, J. et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 25, 2044–2052.e5 (2018).
Cheng, H. Y. et al. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Invest. 126, 3236–3246 (2016).
Westerterp, M. et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 25, 1294–1304.e6 (2017).
Ketelhuth, D. F. J. The immunometabolic role of indoleamine 2,3-dioxygenase in atherosclerotic cardiovascular disease: immune homeostatic mechanisms in the artery wall. Cardiovasc. Res. 115, 1408–1415 (2019).
Polyzos, K. A. et al. Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe−/− mice. Cardiovasc. Res. 106, 295–302 (2015).
Cole, J. E. et al. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc. Natl Acad. Sci. USA 112, 13033–13038 (2015).
Metghalchi, S. et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab. 22, 460–471 (2015).
Sun, J. et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation 122, 808–820 (2010).
Wigren, M. et al. Lack of ability to present antigens on major histocompatibility complex class II molecules aggravates atherosclerosis in ApoE−/− mice. Circulation 139, 2554–2566 (2019).
Bonacina, F. et al. Myeloid apolipoprotein E controls dendritic cell antigen presentation and T cell activation. Nat. Commun. 9, 3083 (2018).
Peshkova, I. O., Fatkhullina, A. R., Mikulski, Z., Ley, K. & Koltsova, E. K. IL-27R signaling controls myeloid cells accumulation and antigen-presentation in atherosclerosis. Sci. Rep. 7, 2255 (2017).
Tay, C. et al. Follicular B cells promote atherosclerosis via T cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 38, e71–e84 (2018).
Williams, J. W. et al. B cell-mediated antigen presentation through MHC class II Is dispensable for atherosclerosis progression. Immunohorizons 3, 37–44 (2019).
Hilgendorf, I. et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129, 1677–1687 (2014).
Acknowledgements
The authors’ work is funded by the US National Institutes of Health (HL136275, HL140976, HL145241, HL146134 and HL148094 to K.L.), a Conrad Prebys Foundation award, a postdoctoral fellowship from the American Heart Association (to M.O.) and the Tullie and Rickey Families SPARK Awards for Innovations in Immunology at La Jolla Institute for Immunology (to P.R. and M.O.).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
K.L. is a co-founder of Atherovax. M.O. and K.L. are named as co-inventors on patents applied for by La Jolla Institute for Immunology relating to cardiovascular diagnostics and therapeutics, and might have the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics. P.R. declares no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks K. Moore and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Major adverse cardiovascular events
-
(MACEs). A composite end point frequently used in cardiovascular research that may include clinical complications such as myocardial infarction, stroke, heart failure, need for coronary revascularization, recurrent angina and death from cardiovascular disease.
- Low-density lipoprotein
-
(LDL). A group of apolipoprotein B-containing macromolecular lipid carriers in the blood that transport cholesterol and triglycerides from the liver to other body tissues.
- Atherothrombosis
-
The state of pathological complication when erosion or rupture of atherosclerotic plaques leads to thrombosis or clot formation, resulting in stroke or myocardial infarction.
- Atherogenesis
-
The process of atherosclerotic plaque formation in the intimal layers of the artery, which is mediated by chronic inflammation and lipid deposition in the vessel walls.
- Apolipoprotein B
-
(APOB). The amphipathic protein backbone of most lipid-transport particles in the plasma, including very-low-density lipoprotein, low-density lipoprotein and chylomicrons.
- Efferocytosis
-
The process by which apoptotic cells are engulfed and cleared by phagocytic cells.
- Classical monocytes
-
Ly-6C+ monocytes in mice and the CD14hiCD16− subset in humans. They are CCR2hiCX3CR1low and are important mediators of tissue inflammation.
- Non-classical monocytes
-
Ly-6C− monocytes in mice and the CD14lowCD16+ subset in humans. They are CCR2lowCX3CR1hi and patrol the vessel walls to maintain endothelial integrity and homeostasis.
- High-density lipoprotein
-
This is the only group of lipoproteins that do not contain apolipoprotein B. High-density lipoprotein is involved in reverse cholesterol transport, delivering excess cholesterol from tissues to the liver.
- Inflammasome
-
A multiprotein cytosolic complex containing members of the NOD-like receptor (NLR) family (such as NLRP3) that integrates signals from several pattern recognition receptors and results in the maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18.
- Trained immunity
-
The reprogramming of innate immune cells, mostly through epigenetic modifications, that creates a ‘memory’ of the initial insult and generates long-lasting innate immunity to specific triggers.
- Very-low-density lipoprotein
-
The primary transporter of endogenous triglycerides from the liver to other tissues in the body.
- Peripherally induced Treg cells
-
Regulatory T (Treg) cells that arise outside the thymus from conventional T cells that acquire FOXP3 expression in response to various stimuli. Unlike thymus-derived Treg cells, peripherally induced Treg cells do not express neuropilin 1.
- Tertiary lymphoid organs
-
Lymphoid structures that form in peripheral tissues in response to chronic inflammation and that have functional and morphological similarities with secondary lymphoid organs.
- Molecular mimicry
-
The possible cross-reactive activation of autoreactive B cells or T cells that are specific for self-derived epitopes that have sequence similarity or structural homology with pathogen-derived foreign epitopes.
Rights and permissions
About this article
Cite this article
Roy, P., Orecchioni, M. & Ley, K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol 22, 251–265 (2022). https://doi.org/10.1038/s41577-021-00584-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00584-1
This article is cited by
-
Breaking tolerance: the autoimmune aspect of atherosclerosis
Nature Reviews Immunology (2024)
-
Thylakoid engineered M2 macrophage for sonodynamic effect promoted cell therapy of early atherosclerosis
Nano Research (2024)
-
Significance of neutrophil extracellular traps-related gene in the diagnosis and classification of atherosclerosis
Apoptosis (2024)
-
Transcriptomic analysis reveals molecular characterization and immune landscape of PANoptosis-related genes in atherosclerosis
Inflammation Research (2024)
-
TREM2 protects from atherosclerosis by limiting necrotic core formation
Nature Cardiovascular Research (2024)