Abstract
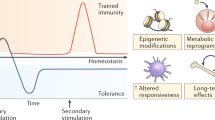
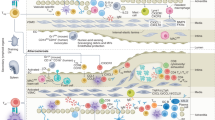
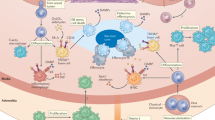
Trained immunity, also known as innate immune memory, is a persistent hyper-responsive functional state of innate immune cells. Accumulating evidence implicates trained immunity as an underlying mechanism of chronic inflammation in atherosclerotic cardiovascular disease. In this context, trained immunity is induced by endogenous atherosclerosis-promoting factors, such as modified lipoproteins or hyperglycaemia, causing broad metabolic and epigenetic reprogramming of the myeloid cell compartment. In addition to traditional cardiovascular risk factors, lifestyle factors, including unhealthy diets, sedentary lifestyle, sleep deprivation and psychosocial stress, as well as inflammatory comorbidities, have been shown to activate trained immunity-like mechanisms in bone marrow haematopoietic stem cells. In this Review, we discuss the molecular and cellular mechanisms of trained immunity, its systemic regulation through haematopoietic progenitor cells in the bone marrow, and the activation of these mechanisms by cardiovascular disease risk factors. We also highlight other trained immunity features that are relevant for atherosclerotic cardiovascular disease, including the diverse cell types that show memory characteristics and transgenerational inheritance of trained immunity traits. Finally, we propose potential strategies for the therapeutic modulation of trained immunity to manage atherosclerotic cardiovascular disease.
Key points
-
Trained immunity denotes the long-term hyperinflammatory functional reprogramming of innate immune cells, which can occur in mature cells and their bone marrow myeloid progenitors.
-
Lifestyle-related factors that promote atherosclerotic cardiovascular disease, including dyslipidaemia, hyperglycaemia, consumption of Western-type diets, obesity, stress and disturbed sleep, have been shown to induce trained immunity in experimental models.
-
Trained immunity is mediated by the activation of intracellular metabolic pathways, including glycolysis, glutaminolysis and mevalonate synthesis, which subsequently induces persistent epigenetic reprogramming of the cell by affecting the activity of epigenetic enzymes.
-
Local histone methylation occurs on genes encoding inflammatory factors, mediated by the action of a class of long non-coding RNAs, called immune gene-priming long non-coding RNAs, that operate within discrete topologically associated domains.
-
We propose trained immunity as a novel framework to understand the role of inflammation in cardiovascular disease pathophysiology, which provides novel pharmacological targets to prevent cardiovascular disease.
-
The key to developing successful treatment strategies focused on trained immunity is to target molecular targets that are specific for trained immunity in specific myeloid cells (or subsets of cells prone to trained immunity) in restricted time windows of trained immunity activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Visseren, F. L. J. et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 42, 3227–3337 (2021).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Bekkering, S. et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis 254, 228–236 (2016).
Shirai, T. et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213, 337–354 (2016).
Li, Y. et al. A functional genomics approach to understand variation in cytokine production in humans. Cell 167, 1099–1110.e14 (2016).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Dominguez-Andres, J. et al. Trained immunity: adaptation within innate immune mechanisms. Physiol. Rev. 103, 313–346 (2023).
Netea, M. G., Quintin, J. & van der Meer, J. W. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Fanucchi, S., Dominguez-Andres, J., Joosten, L. A. B., Netea, M. G. & Mhlanga, M. M. The intersection of epigenetics and metabolism in trained immunity. Immunity 54, 32–43 (2021).
Divangahi, M. et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol. 22, 2–6 (2021).
Higgins, J. P. et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ 355, i5170 (2016).
Prentice, S. et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect. Dis. 21, 993–1003 (2021).
Lanz-Mendoza, H. & Contreras-Garduno, J. Innate immune memory in invertebrates: concept and potential mechanisms. Dev. Comp. Immunol. 127, 104285 (2022).
Bekkering, S. et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1731–1738 (2014).
Thiem, K. et al. Hyperglycemic memory of innate immune cells promotes in vitro proinflammatory responses of human monocytes and murine macrophages. J. Immunol. 206, 807–813 (2021).
Edgar, L. et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 144, 961–982 (2021).
van der Heijden, C. et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ. Res. 127, 269–283 (2020).
Li, X. et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185, 1709–1727.e18 (2022).
Chavakis, T., Wielockx, B. & Hajishengallis, G. Inflammatory modulation of hematopoiesis: linking trained immunity and clonal hematopoiesis with chronic disorders. Annu. Rev. Physiol. 84, 183–207 (2022).
Mitroulis, I., Hajishengallis, G. & Chavakis, T. Bone marrow inflammatory memory in cardiometabolic disease and inflammatory comorbidities. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvad003 (2023).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190.e19 (2018).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175.e14 (2018).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Cheng, S. C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Keating, S. T. et al. Rewiring of glucose metabolism defines trained immunity induced by oxidized low-density lipoprotein. J. Mol. Med. 98, 819–831 (2020).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146.e9 (2018).
Keating, S. T. et al. The Set7 lysine methyltransferase regulates plasticity in oxidative phosphorylation necessary for trained immunity induced by β-glucan. Cell Rep. 31, 107548 (2020).
Groh, L. A. et al. oxLDL-induced trained immunity is dependent on mitochondrial metabolic reprogramming. Immunometabolism 3, e210025 (2021).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Novakovic, B. et al. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368.e14 (2016).
Fok, E. T., Davignon, L., Fanucchi, S. & Mhlanga, M. M. The lncRNA connection between cellular metabolism and epigenetics in trained immunity. Front. Immunol. 9, 3184 (2018).
Fanucchi, S. et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 51, 138–150 (2019).
Mourits, V. P. et al. Lysine methyltransferase G9a is an important modulator of trained immunity. Clin. Transl. Immunol. 10, e1253 (2021).
Moorlag, S. et al. An integrative genomics approach identifies KDM4 as a modulator of trained immunity. Eur. J. Immunol. 52, 431–446 (2022).
Bannister, S. et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci. Adv. 8, eabn4002 (2022).
Verma, D. et al. Anti-mycobacterial activity correlates with altered DNA methylation pattern in immune cells from BCG-vaccinated subjects. Sci. Rep. 7, 12305 (2017).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
McAlpine, C. S. et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Noz, M. et al. Reprogramming of bone marrow myeloid progenitor cells in patients with severe coronary artery disease. eLife 9, e60939 (2020).
Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364, 937–952 (2004).
Bekkering, S. et al. In vitro experimental model of trained innate immunity in human primary monocytes. Clin. Vaccin. Immunol. 23, 926–933 (2016).
van der Valk, F. M. et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 134, 611–624 (2016).
Bekkering, S. et al. Treatment with statins does not revert trained immunity in patients with familial hypercholesterolemia. Cell Metab. 30, 1–2 (2019).
Cosentino, F. et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41, 255–323 (2020).
Miao, F. et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes 63, 1748–1762 (2014).
van der Heijden, C. et al. Aldosterone induces trained immunity: the role of fatty acid synthesis. Cardiovasc. Res. 116, 317–328 (2020).
van der Heijden, C. et al. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 105, e1967–e1980 (2020).
NCD Risk Factor Collaboration (NCD-RisC).Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Schloss, M. J., Swirski, F. K. & Nahrendorf, M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ. Res. 126, 1242–1259 (2020).
Christ, A., Lauterbach, M. & Latz, E. Western diet and the immune system: an inflammatory connection. Immunity 51, 794–811 (2019).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Fuke, N., Nagata, N., Suganuma, H. & Ota, T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors.Nutrients 11, 2277 (2019).
Lopez-Garcia, E. et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 80, 1029–1035 (2004).
van Kampen, E., Jaminon, A., van Berkel, T. J. & Van Eck, M. Diet-induced (epigenetic) changes in bone marrow augment atherosclerosis. J. Leukoc. Biol. 96, 833–841 (2014).
Ifrim, D. C. et al. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vaccin. Immunol. 21, 534–545 (2014).
Geng, S. et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 7, 13436 (2016).
Temba, G. S. et al. Urban living in healthy Tanzanians is associated with an inflammatory status driven by dietary and metabolic changes. Nat. Immunol. 22, 287–300 (2021).
Hata, M. et al. Past history of obesity triggers persistent epigenetic changes in innate immunity and exacerbates neuroinflammation. Science 379, 45–62 (2023).
Dimsdale, J. E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 51, 1237–1246 (2008).
Powell, N. D. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl Acad. Sci. USA 110, 16574–16579 (2013).
Poller, W. C. et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 607, 578–584 (2022).
Barrett, T. J. et al. Chronic stress primes innate immune responses in mice and humans. Cell Rep. 36, 109595 (2021).
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492 (2011).
Vallat, R., Shah, V. D., Redline, S., Attia, P. & Walker, M. P. Broken sleep predicts hardened blood vessels. PLoS Biol. 18, e3000726 (2020).
McAlpine, C. S. et al. Sleep exerts lasting effects on hematopoietic stem cell function and diversity.J. Exp. Med. 219, e20220081 (2022).
Noz, M. P. et al. Sixteen-week physical activity intervention in subjects with increased cardiometabolic risk shifts innate immune function towards a less proinflammatory state. J. Am. Heart Assoc. 8, e013764 (2019).
Leentjens, J. et al. Trained innate immunity as a novel mechanism linking infection and the development of atherosclerosis. Circ. Res. 122, 664–669 (2018).
Arts, R. J. W., Joosten, L. A. B. & Netea, M. G. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front. Immunol. 9, 298 (2018).
Badii, M. et al. Urate-induced epigenetic modifications in myeloid cells. Arthritis Res. Ther. 23, 202 (2021).
Neidhart, M. et al. Oligomeric S100A4 is associated with monocyte innate immune memory and bypass of tolerance to subsequent stimulation with lipopolysaccharides. Front. Immunol. 10, 791 (2019).
Noz, M. P. et al. An explorative study on monocyte reprogramming in the context of periodontitis in vitro and in vivo. Front. Immunol. 12, 695227 (2021).
Crisan, T. O. et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 75, 755–762 (2016).
Zhang, B. et al. Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes.J. Clin. Invest. 137, e147719 (2022).
Yao, Y. et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175, 1634–1650.e17 (2018).
Mitroulis, I., Hajishengallis, G. & Chavakis, T. Trained immunity and cardiometabolic disease: the role of bone marrow. Arterioscler. Thromb. Vasc. Biol. 41, 48–54 (2021).
Moorlag, S. et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 33, 108387 (2020).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161.e12 (2018).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334 (2020).
Hole, C. R. et al. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 10, 2955 (2019).
Kleinnijenhuis, J. et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155, 213–219 (2014).
Schnack, L. et al. Mechanisms of trained innate immunity in oxLDL primed human coronary smooth muscle cells. Front. Immunol. 10, 13 (2019).
Sohrabi, Y. et al. OxLDL-mediated immunologic memory in endothelial cells. J. Mol. Cell Cardiol. 146, 121–132 (2020).
Kalafati, L., Hatzioannou, A., Hajishengallis, G. & Chavakis, T. The role of neutrophils in trained immunity. Immunol. Rev. 314, 142–157 (2023).
Silvestre-Roig, C., Braster, Q., Ortega-Gomez, A. & Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 17, 327–340 (2020).
El-Osta, A. et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 205, 2409–2417 (2008).
Okabe, J. et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ. Res. 110, 1067–1076 (2012).
Katzmarski, N. et al. Transmission of trained immunity and heterologous resistance to infections across generations. Nat. Immunol. 22, 1382–1390 (2021).
Kaufmann, E. et al. Lack of evidence for intergenerational inheritance of immune resistance to infections. Nat. Immunol. 23, 203–207 (2022).
Berendsen, M. et al. Parental Bacillus Calmette-Guerin vaccine scars decrease infant mortality in the first six weeks of life: a retrospective cohort study. EClinicalMedicine 39, 101049 (2021).
Schulz, L. C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl Acad. Sci. USA 107, 16757–16758 (2010).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Mulder, W. J. M., Ochando, J., Joosten, L. A. B., Fayad, Z. A. & Netea, M. G. Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov. 18, 553–566 (2019).
Lawler, P. R. et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur. Heart J. 42, 113–131 (2021).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Courties, G. et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ. Res. 116, 407–417 (2015).
van Leent, M. M. T. et al. Regulating trained immunity with nanomedicine. Nat. Rev. Mater. 7, 465–481 (2022).
Mulder, W. J. M. et al. High-density lipoprotein nanobiologics for precision medicine. Acc. Chem. Res. 51, 127–137 (2018).
van Leent, M. M. T. et al. A modular approach toward producing nanotherapeutics targeting the innate immune system. Sci. Adv. 7, eabe7853 (2021).
Duivenvoorden, R. et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 5, 3065 (2014).
Tang, J. et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation.Sci Adv. 1, e1400223 (2015).
Binderup, T. et al. Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci. Transl. Med. 11, eaaw7736 (2019).
van Leent, M. M. T. et al. Prosaposin mediates inflammation in atherosclerosis. Sci. Transl. Med. 13, eabe1433 (2021).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158 (2014).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Stiekema, L. C. A. et al. Impact of cholesterol on proinflammatory monocyte production by the bone marrow. Eur. Heart J. 42, 4309–4320 (2021).
Seijkens, T. et al. Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. FASEB J. 28, 2202–2213 (2014).
Noye, E. C. et al. Postnatal inflammation in ApoE-/- mice is associated with immune training and atherosclerosis. Clin. Sci. 135, 1859–1871 (2021).
Bekkering, S. et al. Postnatal inflammation following intrauterine inflammation exacerbates the development of atherosclerosis in ApoE-/- mice. Clin. Sci. 133, 1185–1196 (2019).
Huijser, E. et al. Trained immunity in primary Sjogren’s syndrome: linking type I interferons to a pro-atherogenic phenotype. Front. Immunol. 13, 840751 (2022).
Mylona, E. E. et al. Enhanced interleukin-1β production of PBMCs from patients with gout after stimulation with Toll-like receptor-2 ligands and urate crystals. Arthritis Res. Ther. 14, R158 (2012).
Acknowledgements
N.P.R. and M.G.N. were supported by a CVON grant of the Dutch Heart Foundation (IN CONTROL II; CVON2018-27). N.P.R. was supported by a grant of the ERA-CVD Joint Transnational Call 2018, which is supported by the Dutch Heart Foundation in the Hague (JTC2018, project MEMORY; 2018T093). M.G.N. is supported by an ERC Advanced Grant (FP/2007-2013/ERC grant 2012-322698) and a Spinoza Prize (NWO SPI 92-266). S.B. is supported by the Dutch Heart Foundation (2018T028). W.J.M. is supported by an ERC Advanced grant (TOLERANCE) and an NWO Vici grant. Figures for initial submission were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.G.N. and W.J.M. are scientific cofounders of and have equity in Trained Therapeutix Discovery and are scientific founders of BioTRIP. M.G.N. is scientific founder of Lemba. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Ziad Mallat and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riksen, N.P., Bekkering, S., Mulder, W.J.M. et al. Trained immunity in atherosclerotic cardiovascular disease. Nat Rev Cardiol 20, 799–811 (2023). https://doi.org/10.1038/s41569-023-00894-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00894-y
This article is cited by
-
Uncovering atherosclerotic cardiovascular disease by PET imaging
Nature Reviews Cardiology (2024)