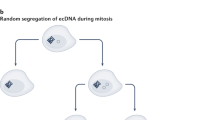
Abstract
Extrachromosomal DNA (ecDNA) has recently been recognized as a major contributor to cancer pathogenesis that is identified in most cancer types and is associated with poor outcomes. When it was discovered over 60 years ago, ecDNA was considered to be rare, and its impact on tumour biology was not well understood. The application of modern imaging and computational techniques has yielded powerful new insights into the importance of ecDNA in cancer. The non-chromosomal inheritance of ecDNA during cell division results in high oncogene copy number, intra-tumoural genetic heterogeneity and rapid tumour evolution that contributes to treatment resistance and shorter patient survival. In addition, the circular architecture of ecDNA results in altered patterns of gene regulation that drive elevated oncogene expression, potentially enabling the remodelling of tumour genomes. The generation of clusters of ecDNAs, termed ecDNA hubs, results in interactions between enhancers and promoters in trans, yielding a new paradigm in oncogenic transcription. In this Review, we highlight the rapid advancements in ecDNA research, providing new insights into ecDNA biogenesis, maintenance and transcription and its role in promoting tumour heterogeneity. To conclude, we delve into a set of unanswered questions whose answers will pave the way for the development of ecDNA targeted therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cox, D., Yuncken, C. & Spriggs, A. Minute chromatin bodies in malignant tumours of childhood. Lancet 286, 55–58 (1965).
Spriggs, A. I., Boddington, M. M. & Clarke, C. M. Chromosomes of human cancer cells. Br. Med. J. 2, 1431 (1962).
Hoff, D. D. V., Needham-VanDevanter, D. R., Yucel, J., Windle, B. E. & Wahl, G. M. Amplified human MYC oncogenes localized to replicating submicroscopic circular DNA molecules. Proc. Natl Acad. Sci. USA 85, 4804–4808 (1988).
Wu, S. et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature 575, 699–703 (2019). This study utilize a multidisciplinary approach combining ultrastructural imaging, long-range optical mapping and computational analysis of whole-genome sequencing to reveal that oncogenes carried on ecDNA in cancer have high expression, owing to the enhanced accessibility of ecDNA and ultra-long-range active chromatin contacts.
Kim, H. et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat. Genet. 52, 891–897 (2020). This study, based on computational analysis of WGS data from 3,212 patients with cancer, reveals that ecDNA amplification is a common phenomenon in various cancer types that results in enhanced oncogene transcription, chromatin accessibility and poor patient survival.
Yi, E. et al. Live-cell imaging shows uneven segregation of extrachromosomal DNA elements and transcriptionally active extrachromosomal DNA hubs in cancer. Cancer Discov. 12, 468–483 (2022).
Lange, J. T. et al. The evolutionary dynamics of extrachromosomal DNA in human cancers. Nat. Genet. 54, 1527–1533 (2022). This study demonstrates that the random inheritance of ecDNA in cancer leads to significant intra-tumoural ecDNA copy number diversity, enabling rapid adaptation to metabolic stresses and targeted therapies, thereby contributing to the aggressive behaviour of ecDNA-containing cancers and underscoring the clinical impact of non-chromosomal oncogene inheritance.
Kaufman, R. J., Brown, P. C. & Schimke, R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc. Natl Acad. Sci. USA 76, 5669–5673 (1979).
Takayama, S. & Uwaike, Y. Analysis of the replication mode of double minutes using the PCC technique combined with BrdUrd labeling. Chromosoma 97, 198–203 (1988).
Barker, P. E. & Hsu, T. C. Are double minutes chromosomes? Exp. Cell Res. 113, 457–458 (1978).
de Salum, S. B. & Larripa, I. Brief communication: minute chromatin bodies in a murine in vitro cell line. J. Natl Cancer Inst. 55, 717–720 (1975).
Ruiz, J. C., Choi, K. H., Hoff, D. D., von, Roninson, I. B. & Wahl, G. M. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line. Mol. Cell. Biol. 9, 109–115 (1989).
Carroll, S. M. et al. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol. Cell. Biol. 8, 1525–1533 (1988).
Turner, K. M. et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 543, 122–125 (2017). Through WGS and cytogenic analyses of thousands of samples from 17 different cancer types, this paper reveals the wide prevalence of ecDNA in cancer and hints that ecDNA-mediated oncogene amplification is a driving force underlying tumour heterogeneity.
Levan, A. & Levan, G. Have double minutes functioning centromeres? Hereditas 88, 81–92 (1978).
Hung, K. L. et al. Targeted profiling of human extrachromosomal DNA by CRISPR-CATCH. Nat. Genet. 54, 1746–1754 (2022).
Luebeck, J. et al. Extrachromosomal DNA in the cancerous transformation of Barrett’s oesophagus. Nature 616, 798–805 (2023). This study, based on WGS data from patients with oesophageal adenocarcinoma and Barrett’s oesophagus, reveals that ecDNA can develop at early stages in the transition from dysplasia to cancer and that the frequency of ecDNA increases as the disease progresses.
Hung, K. L. et al. ecDNA hubs drive cooperative intermolecular oncogene expression. Nature 600, 731–736 (2021). This study demonstrates that ecDNA forms hubs within the nucleus, facilitating intermolecular enhancer–gene interactions that drive oncogene overexpression in various cancer cell types and primary tumours.
Helmsauer, K. et al. Enhancer hijacking determines extrachromosomal circular MYCN amplicon architecture in neuroblastoma. Nat. Commun. 11, 5823 (2020).
Brown, P. C., Beverley, S. M. & Schimke, R. T. Relationship of amplified dihydrofolate reductase genes to double minute chromosomes in unstably resistant mouse fibroblast cell lines. Mol. Cell. Biol. 1, 1077–1083 (1981).
Haber, D. A. & Schimke, R. T. Unstable amplification of an altered dihydrofolate reductase gene associated with double-minute chromosomes. Cell 26, 355–362 (1981).
Kaufman, R. J., Brown, P. C. & Schimke, R. T. Loss and stabilization of amplified dihydrofolate reductase genes in mouse sarcoma s-180 cell lines. Mol. Cell. Biol. 1, 1084–1093 (1981).
Wahl, G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 49, 1333–1340 (1989).
Ruiz, J. C. & Wahl, G. M. Chromosomal destabilization during gene amplification. Mol. Cell. Biol. 10, 3056–3066 (1990).
Alitalo, K., Schwab, M., Lin, C. C., Varmus, H. E. & Bishop, J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-Myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc. Natl Acad. Sci. USA 80, 1707–1711 (1983).
Nathanson, D. A. et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 343, 72–76 (2014).
Hung, K. L., Mischel, P. S. & Chang, H. Y. Gene regulation on extrachromosomal DNA. Nat. Struct. Mol. Biol. 29, 736–744 (2022).
Li, Z., Wang, B., Liang, H. & Han, L. Pioneering insights of extrachromosomal DNA (ecDNA) generation, action and its implications for cancer therapy. Int. J. Biol. Sci. 18, 4006–4025 (2022).
Abeysinghe, H. R., Cedrone, E., Tyan, T., Xu, J. & Wang, N. Amplification of C-MYC as the origin of the homogeneous staining region in ovarian carcinoma detected by micro-FISH. Cancer Genet. Cytogenet. 114, 136–143 (1999).
Storlazzi, C. T. et al. Gene amplification as double minutes or homogeneously staining regions in solid tumors: origin and structure. Genome Res. 20, 1198–1206 (2010).
Benner, S. E., Wahl, G. M. & Hoff, D. D. V. Double minute chromosomes and homogeneously staining regions in tumors taken directly from patients versus in human tumor cell lines. Anti-Cancer Drugs 2, 11–26 (1991).
Kohl, N. E. et al. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell 35, 359–367 (1983).
Bigner, S. H., Mark, J. & Bigner, D. D. Cytogenetics of human brain tumors. Cancer Genet. Cytogenet. 47, 141–154 (1990).
Yoshimoto, M. et al. MYCN gene amplification identification of cell populations containing double minutes and homogeneously staining regions in neuroblastoma tumors. Am. J. Pathol. 155, 1439–1443 (1999).
Vicario, R. et al. Patterns of HER2 gene amplification and response to anti-HER2 therapies. PloS ONE 10, e0129876 (2015).
McGill, J. R. et al. Double minutes are frequently found in ovarian carcinomas. Cancer Genet. Cytogenet. 71, 125–131 (1993).
Purshouse, K. et al. Oncogene expression from extrachromosomal DNA is driven by copy number amplification and does not require spatial clustering in glioblastoma stem cells. eLife 11, e80207 (2022).
Zhou, R. W. & Parsons, R. E. Etiology of super-enhancer reprogramming and activation in cancer. Epigenet. Chromatin 16, 29 (2023).
Morton, A. R. et al. Functional enhancers shape extrachromosomal oncogene amplifications. Cell 179, 1330–1341.e13 (2019). This research highlights the importance of co-amplification of non-coding DNA regions beyond the borders of oncogenes in multiple cancer types.
Koche, R. P. et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat. Genet. 52, 29–34 (2020). This study investigates the landscape of extrachromosomal circular DNA in neuroblastoma, revealing a comprehensive catalogue of somatically acquired circular DNAs that contribute to oncogenic remodelling through chimeric circularization and reintegration into the linear genome.
Weiser, N. E., Hung, K. L. & Chang, H. Y. Oncogene convergence in extrachromosomal DNA hubs. Cancer Discov. 12, OF1–OF4 (2022).
Cremer, T. & Cremer, M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2, a003889 (2010).
Zhu, Y. et al. Oncogenic extrachromosomal DNA functions as mobile enhancers to globally amplify chromosomal transcription. Cancer Cell 39, 694–707.e7 (2021).
Zhu, Y., Gong, L. & Wei, C.-L. Guilt by association: ecDNA as a mobile transactivator in cancer. Trends Cancer 8, 747–758 (2022).
Vendramin, R., Litchfield, K. & Swanton, C. Cancer evolution: Darwin and beyond. EMBO J. 40, e108389 (2021).
Lacina, L. et al. Evolution of cancer progression in the context of Darwinism. Anticancer Res. 39, 1–16 (2019).
Comaills, V. & Castellano-Pozo, M. Chromosomal instability in genome evolution: from cancer to macroevolution. Biology 12, 671 (2023).
Bailey, C., Shoura, M. J., Mischel, P. S. & Swanton, C. Extrachromosomal DNA — relieving heredity constraints, accelerating tumour evolution. Ann. Oncol. 31, 884–893 (2020).
deCarvalho, A. C. et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat. Genet. 50, 708–717 (2018).
Xu, K. et al. Structure and evolution of double minutes in diagnosis and relapse brain tumors. Acta Neuropathol. 137, 123–137 (2019).
Shoshani, O. et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 591, 137–141 (2021). This study reveals that chromothripsis is a major driver of circular ecDNA generation.
Chang, L. et al. Single‐cell third‐generation sequencing‐based multi‐omics uncovers gene expression changes governed by ecDNA and structural variants in cancer cells. Clin. Transl. Med. 13, e1351 (2023).
Sanborn, J. Z. et al. Double minute chromosomes in glioblastoma multiforme are revealed by precise reconstruction of oncogenic amplicons. Cancer Res. 73, 6036–6045 (2013).
Deshpande, V. et al. Exploring the landscape of focal amplifications in cancer using AmpliconArchitect. Nat. Commun. 10, 392 (2019).
Bergstrom, E. N. et al. Mapping clustered mutations in cancer reveals APOBEC3 mutagenesis of ecDNA. Nature 602, 510–517 (2022).
Dharanipragada, P. et al. Blocking genomic instability prevents acquired resistance to MAPK inhibitor therapy in melanoma. Cancer Discov. 13, 880–909 (2023).
González, R. C. et al. Parallel sequencing of extrachromosomal circular DNAs and transcriptomes in single cancer cells. Nat. Genet. 55, 880–890 (2023).
Vogt, N. et al. Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc. Natl Acad. Sci. USA 101, 11368–11373 (2004).
Roy, N. V. et al. Translocation–excision–deletion–amplification mechanism leading to nonsyntenic coamplification of MYC and ATBF1. Genes Chromosom. Cancer 45, 107–117 (2006).
L′Abbate, A. et al. MYC-containing amplicons in acute myeloid leukemia: genomic structures, evolution, and transcriptional consequences. Leukemia 32, 2152–2166 (2018).
L’Abbate, A. et al. Genomic organization and evolution of double minutes/homogeneously staining regions with MYC amplification in human cancer. Nucleic Acids Res. 42, 9131–9145 (2014).
Zuberi, L., Adeyinka, A. & Kuriakose, P. Rapid response to induction in a case of acute promyelocytic leukemia with MYC amplification on double minutes at diagnosis. Cancer Genet. Cytogenet. 198, 170–172 (2010).
Yang, L. et al. Diverse mechanisms of somatic structural variations in human cancer genomes. Cell 153, 919–929 (2013).
Møller, H. D. et al. CRISPR-C: circularization of genes and chromosome by CRISPR in human cells. Nucleic Acids Res. 46, gky767 (2018).
Gaillard, H., García-Muse, T. & Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 15, 276–289 (2015).
Lee, J. A., Carvalho, C. M. B. & Lupski, J. R. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131, 1235–1247 (2007).
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Ly, P. et al. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat. Genet. 51, 705–715 (2019).
Nones, K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 5, 5224 (2014).
Rausch, T. et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 148, 59–71 (2012).
Rosswog, C. et al. Chromothripsis followed by circular recombination drives oncogene amplification in human cancer. Nat. Genet. 53, 1673–1685 (2021).
Murnane, J. P. Telomere dysfunction and chromosome instability. Mutat. Res. Fundam. Mol. Mech. Mutagen. 730, 28–36 (2012).
Gisselsson, D. et al. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc. Natl Acad. Sci. USA 97, 5357–5362 (2000).
Lee, J. J.-K. et al. ERα-associated translocations underlie oncogene amplifications in breast cancer. Nature 618, 1024–1032 (2023). This study investigates the origin of focal copy-number amplifications, a common oncogenic event, in breast cancer, revealing a mechanism termed translocation–bridge amplification, which involves inter-chromosomal translocations that lead to dicentric chromosome bridge formation and breakage.
Balaban-Malenbaum, G. & Gilbert, F. Double minute chromosomes and the homogeneously staining regions in chromosomes of a human neuroblastoma cell line. Science 198, 739–741 (1977).
Lo, A. W. L. et al. DNA amplification by breakage/fusion/bridge cycles initiated by spontaneous telomere loss in a human cancer cell line. Neoplasia 4, 531–538 (2002).
Barker, P. E., Drwinga, H. L., Hittelman, W. N. & Maddox, A.-M. Double minutes replicate once during S phase of the cell cycle. Exp. Cell Res. 130, 353–360 (1980).
Lima-de-Faria, A. & Jaworska, H. Late DNA synthesis in heterochromatin. Nature 217, 138–142 (1968).
Itoh, N. & Shimizu, N. DNA replication-dependent intranuclear relocation of double minute chromatin. J. Cell Sci. 111, 3275–3285 (1998).
Snapka, R. M. & Varshavsky, A. Loss of unstably amplified dihydrofolate reductase genes from mouse cells is greatly accelerated by hydroxyurea. Proc. Natl Acad. Sci. USA 80, 7533–7537 (1983).
Kaufman, R. J. & Schimke, R. T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol. Cell. Biol. 1, 1069–1076 (1981).
Kanda, T., Sullivan, K. F. & Wahl, G. M. Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8, 377–385 (1998).
Kanda, T., Otter, M. & Wahl, G. M. Mitotic segregation of viral and cellular acentric extrachromosomal molecules by chromosome tethering. J. Cell Sci. 114, 49–58 (2001).
Hamkalo, B. A., Farnham, P. J., Johnston, R. & Schimke, R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc. Natl Acad. Sci. USA 82, 1126–1130 (1985).
Deng, X. et al. Double minute chromosomes in mouse methotrexate-resistant cells studied by atomic force microscopy. Biochem. Biophys. Res. Commun. 346, 1228–1233 (2006).
Trivedi, P., Steele, C. D., Au, F. K. C., Alexandrov, L. B. & Cleveland, D. W. Mitotic tethering enables inheritance of shattered micronuclear chromosomes. Nature 618, 1049–1056 (2023).
Lin, Y.-F. et al. Mitotic clustering of pulverized chromosomes from micronuclei. Nature 618, 1041–1048 (2023).
Bode, J. et al. The hitchhiking principle: optimizing episomal vectors for the use in gene therapy and biotechnology. Gene Ther. Mol. Biol. 6, 33–46 (2001).
Huang, K.-C., Yamasaki, E. F. & Snapka, R. M. Maintenance of episomal SV40 genomes in GM637 human fibroblasts. Virology 262, 457–469 (1999).
Piirsoo, M., Ustav, E., Mandel, T., Stenlund, A. & Ustav, M. Cis and trans requirements for stable episomal maintenance of the BPV‐1 replicator. EMBO J. 15, 1–11 ( (1996).
Ohshima, K., Suzumiya, J., Kanda, M., Kato, A. & Kikuchi, M. Integrated and episomal forms of Epstein–Barr virus (EBV) in EBV associated disease. Cancer Lett. 122, 43–50 (1998).
Beverley, S. M., Coderre, J. A., Santi, D. V. & Schimke, R. T. Unstable DNA amplifications in methotrexate resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell 38, 431–439 (1984).
Saito-Adachi, M. et al. Oncogenic structural aberration landscape in gastric cancer genomes. Nat. Commun. 14, 3688 (2023).
Shimizu, N., Hashizume, T., Shingaki, K. & Kawamoto, J. Amplification of plasmids containing a mammalian replication initiation region is mediated by controllable conflict between replication and transcription. Cancer Res. 63, 5281–5290 (2003).
Shimizu, N., Miura, Y., Sakamoto, Y. & Tsutsui, K. Plasmids with a mammalian replication origin and a matrix attachment region initiate the event similar to gene amplification. Cancer Res. 61, 6987–6990 (2001).
Muraki, K. & Murnane, J. P. The DNA damage response at dysfunctional telomeres, and at interstitial and subtelomeric DNA double-strand breaks. Genes Genet. Syst. 92, 135–152 (2017).
Shimizu, N., Misaka, N., Utani, K. & Nonselective, D. N. A. Damage induced by a replication inhibitor results in the selective elimination of extrachromosomal double minutes from human cancer cells. Genes Chromosomes Cancer 46, 865–874 (2007).
Yu, L. et al. Gemcitabine eliminates double minute chromosomes from human ovarian cancer cells. PloS ONE 8, e71988 (2013).
Hoff, D. D. V. et al. Hydroxyurea accelerates loss of extrachromosomally amplified genes from tumor cells. Cancer Res. 51, 6273–6279 (1991).
Hintzsche, H. et al. Fate of micronuclei and micronucleated cells. Mutat. Res. Rev. Mutat. Res. 771, 85–98 (2017).
Wu, T. et al. Extrachromosomal DNA formation enables tumor immune escape potentially through regulating antigen presentation gene expression. Sci. Rep. 12, 3590 (2022). This study explores the relationship between ecDNA and immune evasion in cancer, finding that the presence of ecDNA is associated with markers of tumour immune evasion, suggesting that cancer cells may use ecDNA as a mechanism to escape immune surveillance.
Groves, I. J. & Coleman, N. Human papillomavirus genome integration in squamous carcinogenesis: what have next‐generation sequencing studies taught us? J. Pathol. 245, 9–18 (2018).
Morgan, I., DiNardo, L. & Windle, B. Integration of human papillomavirus genomes in head and neck cancer: is it time to consider a paradigm shift? Viruses 9, 208 (2017).
Chowdhry S. et al. Tumors driven by oncogene amplified extrachromosomal DNA (ecDNA) demonstrate enhanced sensitivity to cell cycle checkpoint kinase 1 (CHK1) inhibition. Cancer Res. 83, 1626 (2023).
US National Library of Medicine. Study of the CHK1 inhibitor BBI-355, an ecDNA-directed therapy, in subjects with tumors with oncogene amplifications (POTENTIATE). ClinicalTrials.gov https://classic.clinicaltrials.gov/ct2/show/NCT05827614 (2023).
Pradella, D. et al. Immortalization and transformation of primary cells mediated by engineered ecDNAs. Preprint at bioRxiv https://doi.org/10.1101/2023.06.25.546239 (2023).
Sahajpal, N. S., Barseghyan, H., Kolhe, R., Hastie, A. & Chaubey, A. Optical genome mapping as a next-generation cytogenomic tool for detection of structural and copy number variations for prenatal genomic analyses. Genes 12, 398 (2021).
Rajkumar, U. et al. EcSeg: semantic segmentation of metaphase images containing extrachromosomal DNA. iScience 21, 428–435 (2019).
Cohen, S. & Lavi, S. Induction of circles of heterogeneous sizes in carcinogen-treated cells: two-dimensional gel analysis of circular DNA molecules. Mol. Cell. Biol. 16, 2002–2014 (1996).
Kumar, P. et al. ATAC-seq identifies thousands of extrachromosomal circular DNA in cancer and cell lines. Sci. Adv. 6, eaba2489 (2020).
Møller, H. D. et al. Genome-wide purification of extrachromosomal circular DNA from eukaryotic cells. J. Vis. Exp. https://doi.org/10.3791/54239 (2016).
Li, G. et al. Chromatin interaction analysis with paired-end tag (ChIA-PET) sequencing technology and application. BMC Genom. 15, S11 (2014).
Li, F. et al. FLED: a full-length eccDNA detector for long-reads sequencing data. Brief. Bioinform. 24, bbad388 (2023).
Mann, L., Seibt, K. M., Weber, B. & Heitkam, T. ECCsplorer: a pipeline to detect extrachromosomal circular DNA (eccDNA) from next-generation sequencing data. BMC Bioinform. 23, 40 (2022).
Degasperi, A. et al. Substitution mutational signatures in whole-genome-sequenced cancers in the UK population. Science https://doi.org/10.1126/science.abl9283 (2022).
Acknowledgements
This work was delivered as part of the eDyNAmiC team supported by the Cancer Grand Challenges partnership funded by Cancer Research UK (CRUK) (P.M. and H.C., CGCATF-2021/100012) and the National Cancer Institute (P.M. and H.C., OT2CA278688). This study was also supported by a grant from the National Brain Tumour Society (P.S.M.) and National Institutes of Health (NIH) R01-CA238379 (P.S.M.). X.Y. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-2474-22). In addition, we thank members of Chang and Mischel labs for helpful discussions.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
H.C. is a co-founder of Accent Therapeutics, Boundless Bio, Cartography Biosciences and Orbital Therapeutics, and is an adviser to 10x Genomics, Arsenal Biosciences, Chroma Medicine and Spring Discovery. P.M. is a co-founder of, chairs the scientific advisory board (SAB) of and has equity interest in Boundless Bio. P.M. is also an adviser with equity for Asteroid Therapeutics. X.Y declares no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Tatsuhrio Shibata, Andrew Futreal and Anton Henssen for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Mitelman database: https://mitelmandatabase.isb-cgc.org
Glossary
- Breakage–fusion–bridge (BFB) cycle
-
A mechanism of chromosomal instability wherein broken ends of different chromatids or chromosomes fuse.
- ChIA-Drop
-
A chromatin conformation capture technique that combines chromatin immunoprecipitation with droplet-based single-cell sequencing, allowing the investigation of chromatin interactions at the single-cell level.
- Chromatin interaction analysis with paired-end-tag sequencing
-
(ChIA-PET). A genomic technique that enables the identification and mapping of long-range chromatin interactions by combining chromatin immunoprecipitation with paired-end high-throughput sequencing.
- Chromosome territories
-
The specific region of the nucleus that a certain chromosome tends to occupy.
- CRISPR-C
-
A technique that allows in vitro ecDNA generation in cells by inducing double-strand breaks flanking the region of interest upon the delivery of pairs of CRISPR–Cas9 guide RNAs.
- Double minutes
-
(DM). A traditional term for ecDNA that is still indicated in names of most ecDNA-containing cell lines, for example Colo 320DM.
- Gene amplification
-
A process by which the copy number of a specific gene is increased in a cell, leading to heightened expression and contributing to the development and progression of cancer when oncogene is amplified.
- Genetic identity by descent
-
A genetic term indicating the sharing of a specific DNA segment between two or more individuals owing to inheritance from a common ancestor.
- Homogeneously staining region
-
(HSR). A large repetitive region in a chromosome that displays a homogeneous staining pattern when targeted with probes; in this Review, HSR is used to mostly refer to oncogene amplification regions in chromosomes.
- Microhomology
-
The presence of short, identical or nearly identical sequences (typically 2 to 20 base pairs) at or near the ends of two DNA fragments, typically arising during DNA repair or rearrangment to facilitate precise alignment.
- Micronuclei
-
Small, additional nuclei that can form during cell division and contain fragments of chromosomes or entire chromosomes that were not incorporated into the main nucleus.
- Non-homologous end joining
-
(NHEJ). DNA repair mechanism whereby double-stand breaks are ligated without the need for a homologous template.
- Nucleosomes
-
Basic structural units of eukaryotic DNA packaging, consisting of a segment of coiled DNA around eight core histone proteins.
- Repli-seq
-
A genomic technique that involves sequencing the DNA obtained from cells at different stages of the S phase to map DNA replication patterns and identify regions undergoing replication.
- Topologically associated domain
-
(TAD). A genomic region that spatially interacts with itself.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, X., Mischel, P. & Chang, H. Extrachromosomal DNA in cancer. Nat Rev Cancer 24, 261–273 (2024). https://doi.org/10.1038/s41568-024-00669-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-024-00669-8