Abstract
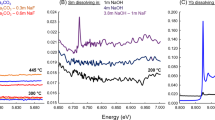
Emerging renewable energy technologies and low-carbon transportation rely heavily on the unique optical and magnetic properties of the rare earth elements. The medium to heavy rare earth elements, neodymium to lutetium, are most sought by industry but are the least abundant in nature. Only a small proportion of known rare earth element deposits are enriched in these elements. Identifying additional sources of medium to heavy rare earth elements for resource exploration requires improved understanding of the mechanisms responsible for the formation of such highly fractionated deposits. Here we report the results of experiments demonstrating a mechanism that could lead to enrichment of medium to heavy rare earth elements in ore-forming hydrothermal systems. In our experiments, we simulated natural hydrothermal systems by heating a column containing apatite and fluorite through which we pumped a chloride-rich solution bearing rare earth elements. Analysis of our experiments shows that the fluoride mineral fluocerite can serve as a precursor phase that fractionates rare earth elements before it is subsequently converted to a thermodynamically more stable mineral. Our findings identify geological settings in which fluocerite is observed or predicted to occur as potential exploration targets for deposits enriched in medium to heavy rare earth elements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data on which this manuscript is based are available to readers from the Supplementary Information. Source data are provided with this paper.
References
Connelly, N. G., Damhus, T., Hartshorn, R. M. & Hutton, A. T. Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (Royal Society of Chemistry, 2005).
Haxel, G. B., Hedrick, J. B. & Orris, G. J. Rare Earth Elements—Critical Resources for High Technology (US Geological Survey, 2002).
Castor, S. B. & Hendrick, J. B. in Industrial Minerals and Rocks: Commodities, Markets, and Uses (eds Kogel, J. E. et al.) 769–792 (Society for Mining Mineralogy, 2006).
Castor, S. B. The Mountain Pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can. Mineral. 46, 779–806 (2008).
Moore, M., Chakhmouradian, A. R., Mariano, A. N. & Sidhu, R. Evolution of rare-earth mineralization in the Bear Lodge carbonatite, Wyoming: mineralogical and isotopic evidence. Ore Geol. Rev. 64, 499–521 (2015).
Gysi, A. P. & Williams-Jones, A. E. Hydrothermal mobilization of pegmatite-hosted REE and Zr at Strange Lake, Canada: a reaction path model. Geochim. Cosmochim. Acta 122, 324–352 (2013).
Salvi, S. & Williams-Jones, A. E. The role of hydrothermal processes in the granite-hosted Zr, Y, REE deposit at Strange Lake, Quebec/Labrador: evidence from fluid inclusions. Geochim. Cosmochim. Acta 54, 2403–2418 (1990).
Chao, E. C. T., Back, J. M., Minkin, J. A. & Yinchen, R. Host-rock controlled epigenetic, hydrothermal metasomatic origin of the Bayan Obo REE Fe–Nb ore deposit, Inner Mongolia, P.R.C. Appl. Geochem. 7, 443–458 (1992).
Deng, M. et al. REE mineralization in the Bayan Obo deposit, China: evidence from mineral paragenesis. Ore Geol. Rev. 91, 100–109 (2017).
Sheard, E. R., Williams-Jones, A. E., Heiligmann, M., Pederson, C. & Trueman, D. L. Controls on the concentration of zirconium, niobium, and the rare earth elements in the Thor Lake rare metal deposit, Northwest Territories, Canada. Econ. Geol. 107, 81–104 (2012).
Vasyukova, O. V. & Williams-Jones, A. E. Fluoride–silicate melt immiscibility and its role in REE ore formation: evidence from the Strange Lake rare metal deposit, Québec-Labrador, Canada. Geochim. Cosmochim. Acta 139, 110–130 (2014).
Möller, V. & Williams-Jones, A. E. Magmatic and hydrothermal controls on the mineralogy of the basal zone, Nechalacho REE-Nb–Zr deposit, Canada. Econ. Geol. 112, 1823–1856 (2017).
Li, M. Y. H., Zhou, M. F. & Williams-Jones, A. E. The genesis of regolith-hosted heavy rare earth element deposits: insights from the world-class Zudong deposit in Jiangxi province, South China. Econ. Geol. 114, 541–568 (2019).
Van Gosen, B. S. & Sengupta, D. in Reviews in Economic Geology (eds Verplanck, P. L. & Hitzman, M. W.) 81–100 (Society of Economic Geologists, 2016).
Dai, S., Graham, I. T. & Ward, C. R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 159, 82–95 (2016).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–766 (1976).
Kanazawa, Y. & Kamitani, M. Rare earth minerals and resources in the world. J. Alloy. Compd. 408–412, 1339–1343 (2006).
Taggart, R. K., Hower, J. C., Dwyer, G. S. & Hsu-Kim, H. Trends in the rare earth element content of U.S.-based coal combustion fly ashes. Environ. Sci. Technol. 50, 5919–5926 (2016).
Williams-Jones, A. E., Wollenberg, R. & Bodeving, S. Hydrothermal fractionation of the rare earth elements and the genesis of the Lofdal REE deposit, Namibia. In Symposium on Critical and Strategic Materials Proceedings (eds Simandl, G. J. & Neetz, M.) 125–130 (British Columbia Ministry of Energy and Mines, 2015).
Richter, L., Diamond, L. W., Atanasova, P., Banks, D. A. & Gutzmer, J. Hydrothermal formation of heavy rare earth element (HREE)–xenotime deposits at 100 °C in a sedimentary basin. Geology 46, 263–266 (2018).
Cook, N. J. et al. Mineral chemistry of rare earth element (REE) mineralization, Browns Ranges, Western Australia. Lithos 172–173, 192–213 (2013).
Banks, D. A., Yardley, B. W. D., Campbell, A. R. & Jarvis, K. E. REE composition of an aqueous magmatic fluid: a fluid inclusion study from the Capitan Pluton, New Mexico, U.S.A. Chem. Geol. 113, 259–272 (1994).
Winter, J. D. Principles of Igneous and Metamorphic Petrology (Pearson, 2013).
Nabyl, Z. et al. A window in the course of alkaline magma differentiation conducive to immiscible REE-rich carbonatites. Geochim. Cosmochim. Acta 282, 297–323 (2020).
Migdisov, A., Williams-Jones, A. E., Brugger, J. & Caporuscio, F. A. Hydrothermal transport, deposition, and fractionation of the REE: experimental data and thermodynamic calculations. Chem. Geol. 439, 13–42 (2016).
Anenburg, M., Mavrogenes, J. A., Frigo, C. & Wall, F. Rare earth element mobility in and around carbonatites controlled by sodium, potassium, and silica. Sci. Adv. 6, eabb6570 (2020).
Vasyukova, O. V. & Williams-Jones, A. E. The evolution of immiscible silicate and fluoride melts: implications for REE ore-genesis. Geochim. Cosmochim. Acta 172, 205–224 (2016).
Siegel, K., Vasyukova, O. V. & Williams-Jones, A. E. Magmatic evolution and controls on rare metal-enrichment of the Strange Lake A-type peralkaline granitic pluton, Québec-Labrador. Lithos 308–309, 34–52 (2018).
Vasyukova, O. V. & Williams-Jones, A. E. Tracing the evolution of a fertile REE granite by modelling amphibole-melt partitioning, the Strange Lake story. Chem. Geol. 514, 79–89 (2019).
Salvi, S. & Williams-Jones, A. E. The role of hydrothermal processes in concentrating HFSE in the Strange Lake peralkaline complex, northeastern Canada. Geochim. Cosmochim. Acta 60, 1917–1932 (1996).
Smith, M. et al. The origin of secondary heavy rare earth element enrichment in carbonatites: constraints from the evolution of the Huanglongpu district, China. Lithos 308–309, 65–82 (2018).
Williams-Jones, A. E., Samson, I. M. & Olivo, G. R. The genesis of hydrothermal fluorite–REE deposits in the Gallinas Mountains, New Mexico. Econ. Geol. 95, 327–341 (2000).
Migdisov, A., Williams-Jones, A. E. & Wagner, T. An experimental study of the solubility and speciation of the rare earth elements (III) in fluoride- and chloride-bearing aqueous solutions at temperatures up to 300 °C. Geochim. Cosmochim. Acta 73, 7087–7109 (2009).
Migdisov, A. & Williams-Jones, A. E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 49, 987–997 (2014).
Migdisov, A. et al. A spectroscopic study of uranyl speciation in chloride-bearing solutions at temperatures up to 250 °C. Geochim. Cosmochim. Acta 222, 130–145 (2018).
Williams-Jones, A. E., Migdisov, A. & Samson, I. M. Hydrothermal mobilisation of the rare earth elements—a tale of ‘ceria’ and ‘yttria’. Elements 8, 355–360 (2012).
Migdisov, A. & Williams-Jones, A. E. A spectrophotometric study of neodymium(III) complexation in chloride solutions. Geochim. Cosmochim. Acta 66, 4311–4323 (2002).
Migdisov, A. & Williams-Jones, A. E. A spectrophotometric study of Nd(III), Sm(III) and Er(III) complexation in sulfate-bearing solutions at elevated temperatures. Geochim. Cosmochim. Acta 72, 5291–5303 (2008).
Migdisov, A., Reukov, V. V. & Williams-Jones, A. E. A spectrophotometric study of neodymium(III) complexation in sulfate solutions at elevated temperatures. Geochim. Cosmochim. Acta 70, 983–992 (2006).
Bunzli, J.-C. G. & McGill, I. in Ullmann’s Encyclopedia of Industrial Chemistry 1–51 (John Wiley and Sons, 2018).
Migdisov, A., Guo, X., Nisbet, H., Xu, H. & Williams-Jones, A. E. Fractionation of REE, U, and Th in natural ore-forming hydrothermal systems: thermodynamic modeling. J. Chem. Thermodyn. 128, 305–319 (2018).
Veksler, I. V. et al. Partitioning of elements between silicate melt and immiscible fluoride, chloride, carbonate, phosphate and sulfate melts, with implications to the origin of natrocarbonatite. Geochim. Cosmochim. Acta 79, 20–40 (2012).
Martin, L. H. J., Schmidt, M. W., Mattsson, H. B. & Guenther, D. Element partitioning between immiscible carbonatite and silicate melts for dry and H2O-bearing systems at 1–3 GPa. J. Petrol. 54, 2301–2338 (2013).
Lahti, S. I. & Suominen, V. Occurrence, crystallography and chemistry of the fluocerite–bastnaesite–cerianite intergrowth from the Fjälskär granite, southwestern Finland. Bull. Geol. Soc. Finl. 60, 45–53 (2017).
Holtstam, D. & Andersson, U. B. The REE minerals of the bastnäs-type deposits, south-central Sweden. Can. Mineral. 45, 1073–1114 (2007).
Beukes, G. J., De Bruiyn, H. & Van Der Westhuizen, W. A. Fluocerite and associated minerals from the Baviaanskranz granite pegmatite near Kakamas, South Africa. South Afr. J. Geol. 94, 313–320 (1991).
Hetherington, C. J., Harlov, D. E. & Budzyń, B. Experimental metasomatism of monazite and xenotime: mineral stability, REE mobility and fluid composition. Mineral. Petrol. 99, 165–184 (2010).
Cherniak, D. J. Pb and rare earth element diffusion in xenotime. Lithos 88, 1–14 (2006).
Dorozhkin, S. V. Dissolution mechanism of calcium apatites in acids: a review of literature. World J. Methodol. 2, 1–17 (2012).
Simmons, S. F. & Brown, K. L. Gold in magmatic hydrothermal solutions and the rapid formation of a giant ore deposit. Science 314, 288–292 (2006).
Zhao, J., Brugger, J. & Pring, A. Mechanism and kinetics of hydrothermal replacement of magnetite by hematite. Geosci. Front. 10, 29–41 (2019).
Putnis, A., Spencer, C. J. & Raimondo, T. Timescales of geological processes: preface. Geosci. Front. 10, 1–3 (2019).
von Quadt, A. et al. Zircon crystallization and the lifetimes of ore-forming magmatic-hydrothermal systems. Geology 39, 731–734 (2011).
Smith, M. P., Henderson, P. & Campbell, L. S. Fractionation of the REE during hydrothermal processes: constraints from the Bayan Obo Fe–REE–Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 64, 3141–3160 (2000).
Voigt, M., Rodriguez-Blanco, J. D., Vallina, B., Benning, L. G. & Oelkers, E. H. An experimental study of hydroxylbastnasite solubility in aqueous solutions at 25 °C. Chem. Geol. 430, 70–77 (2016).
Gysi, A. P., Harlov, D. & Miron, G. D. The solubility of monazite (CePO4), SmPO4, and GdPO4 in aqueous solutions from 100 to 250 °C. Geochim. Cosmochim. Acta 242, 143–164 (2018).
Gysi, A. P., Williams-Jones, A. E. & Harlov, D. The solubility of xenotime-(Y) and other HREE phosphates (DyPO4, ErPO4 and YbPO4) in aqueous solutions from 100 to 250 °C and psat. Chem. Geol. 401, 83–95 (2015).
Acknowledgements
Research presented in this article was supported both by the National Energy Technology Laboratory under project number FE-810-17-FY17 and by the Laboratory Directed Research and Development programme of Los Alamos National Laboratory under project number 20190057DR. A.C.S. and X.G. acknowledge the support by the US Department of Energy Office of Nuclear Energy grants DE-NE0008582 and DE-NE0008689.
Author information
Authors and Affiliations
Contributions
A.M. and H.B. conceived the research. A.C.S. developed the experimental method and conducted experiments. K.S. and K.G.M. performed scanning electron microscopy and μXRF studies, respectively. All co-authors participated in discussions, interpretation of the data and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks John Mavrogenes, Kathryn Goodenough and Kenzo Sanematsu for their contribution to the peer review of this work. Primary Handling Editors: Tamara Goldin and Simon Harold, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods and thermodynamic calculation descriptions, Results, Discussion, Figs. 1–9 and Table 1.
Supplementary Data
The source data that were used to produce the various figures in the main text and supplementary text.
Source data
Source Data Fig. 1
Data used to generate Fig. 1a in the main text and data used to generate Fig. 1b in the main text.
Source Data Fig. 3
Data used to generate Fig. 3a,b in the main text and data used to generate Fig. 3c,d in the main text.
Rights and permissions
About this article
Cite this article
Strzelecki, A.C., Migdisov, A., Boukhalfa, H. et al. Fluocerite as a precursor to rare earth element fractionation in ore-forming systems. Nat. Geosci. 15, 327–333 (2022). https://doi.org/10.1038/s41561-022-00921-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-00921-6
This article is cited by
-
Swelling inhibition and infiltration promotion mechanism of polyethyleneimine
Journal of Central South University (2024)
-
An unique, fluocerite-rich REE deposit in Henan province, Central China: the missing link in magmatic-hydrothermal REE mineralizing systems?
Contributions to Mineralogy and Petrology (2023)