Abstract
Antipsychotic drugs (AP) are highly efficacious treatments for psychiatric disorders but are associated with significant metabolic side-effects. The circadian clock maintains metabolic homeostasis by sustaining daily rhythms in feeding, fasting and hormone regulation but how circadian rhythms interact with AP and its associated metabolic side-effects is not well-known. We hypothesized that time of AP dosing impacts the development of metabolic side-effects. Weight gain and metabolic side-effects were compared in C57Bl/6 mice and humans dosed with APs in either the morning or evening. In mice, AP dosing at the start of the light cycle/rest period (AM) resulted in significant increase in food intake and weight gain compared with equivalent dose before the onset of darkness/active period (PM). Time of AP dosing also impacted circadian gene expression, metabolic hormones and inflammatory pathways and their diurnal expression patterns. We also conducted a retrospective examination of weight and metabolic outcomes in patients who received risperidone (RIS) for the treatment of serious mental illness and observed a significant association between time of dosing and severity of RIS-induced metabolic side-effects. Time restricted feeding (TRF) has been shown in both mouse and some human studies to be an effective therapeutic intervention against obesity and metabolic disease. We demonstrate, for the first time, that TRF is an effective intervention to reduce AP-induced metabolic side effects in mice. These studies identify highly effective and translatable interventions with potential to mitigate AP-induced metabolic side effects.
Similar content being viewed by others
Introduction
Antipsychotic drugs (AP) are highly efficacious treatments for serious mental illnesses (SMI) such as schizophrenia, bipolar disorder and major depression, but frequently cause weight gain and metabolic disease1. The metabolic side-effects of APs exacerbate medical comorbidities and worsen health outcomes in SMI patients, a population already at high risk for cardiometabolic disorders and early mortality2. Moreover, AP-induced metabolic side-effects lead to treatment discontinuation and non-compliance that destabilize SMI and worsen outcomes such as social/occupational impairment and suicide. Both neurobehavioral and peripheral metabolic factors have been implicated as key factors driving adverse effects of AP but the specific molecular mechanisms remain unknown3. Previous studies have identified potential adjuvant drugs that can mitigate some of the weight gain effects of APs4,5,6,7,8,9,10 but these interventions have yet to make a significant impact on the problem associated with APs. Additional strategies are needed to further reduce the metabolic burden of AP in patients with SMI to make these drugs safer, more tolerable and effective.
Circadian rhythms are controlled by multiple centers including the central clock in the hypothalamic suprachiasmatic nucleus (SCN) that regulate ~24 h rhythms in food intake and behavior while the peripheral clocks in the liver, adipose, pancreas, muscle and other metabolically active tissues control circadian rhythms in energy expenditure and whole-body insulin sensitivity11. These central and peripheral circadian rhythms help to optimize energy harvest and utilization across the 24-h dark/light cycle. A transcriptional/ translational feedback loop made up of ~20 “core clock genes” maintains essential functions underlying cellular circadian rhythms. At the center of this loop, the proteins Clock and Bmal1 bind to form a heterodimeric transcriptional activator. The Bmal1/Clock complex drives the expression of period (Per1/2) genes, and cryptochrome (Cry1/2) transcriptional repressors that sustain circadian oscillators with a period length of ~24 h. A second feedback loop provides additional robustness to the oscillatory mechanism and consists of nuclear receptors of the Rev-erbα which drives rhythmic Bmal1 expression. These intricate feedback loops generate rhythms with a period of about a day that maintains metabolic homeostasis by sustaining daily rhythms in feeding and fasting12 such that consuming a higher proportion of calories during the evening increases the likelihood of being overweight in humans13 and similar findings are observed in mice where consuming calories during the light cycle results in greater weight gain compared to mice fed in the dark cycle14. Interestingly, eating within a restricted time window (Time Restricted Feeding (TRF) has been shown in both mouse15,16 and human studies17,18 to be an effective therapeutic intervention against obesity and metabolic disease12,19. Previous work in animal models has demonstrated that AP drugs have effects on signaling in the SCN20,21 and suggest that chronobiological interventions such as melatonin treatment in animals or humans20,22 or adjusting the time of drug dosing23 or feeding14 can reduce weight gain. In addition, some studies have suggested that the hyperglycemic effect of APs may be modulated by dosing time24,25. However, additional work is needed to determine which APs have the strongest time dependent effects and to what extent circadian rhythms interact with drug dosing and feeding to affect metabolism and behaviors governing weight after AP treatment.
In our previous work, we found that the timing of administration of the AP sulpiride affected the weight gain and metabolic outcomes in mice26. Sulpiride acts by selective antagonism of the D2 dopamine receptor whereas most commonly used AP have a more complex mechanism involving additional receptors and are associated with greater risk for weight gain. While almost all APs result in weight gain27, the “second generation” APs such as risperidone (RIS) and olanzapine (OLZ) cause the most weight gain28,29 and are widely prescribed30. In these studies, we evaluated whether dosing time of OLZ or RIS impacted AP-induced weight gain and metabolic side-effects. We then further explored whether TRF can mitigate the metabolic impairments associated with AP-induced weight gain.
Results
Time of dosing impacts AP-induced metabolic side effects in mice
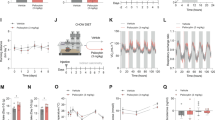
To investigate the impact of time of AP dosing on metabolic side effects, we used a mouse model of AP-induced hypeprhagia and weight gain. Dosing of APs at a specific time of day was achieved through self-administration of AP (RIS or OLZ) mixed into peanut butter pellets31. AP treatment close to the start of the light cycle (AM) resulted in significantly higher food intake (Fig. 1A, B) and weight gain (Fig. 1C, D) compared with CON treatment. In contrast, APs administed at the start of the dark cycle (PM) did not induce significant increases in food intake and body weight compared with CON treatment at that time. Therefore, these data suggest that the hyperphagia and weight gain side-effects of APs are highly dependent on the time of dosing. Two hours after AP treatment, we measured circulating blood glucose and observed increased glucose in RIS-AM (Fig. 1E) treated mice compared with RIS-PM or vehicle-control groups. No significant changes in glucose were observed with OLZ (Fig. 1F). RIS is the more commonly prescribed than OLZ32 and thus we focused on RIS treatment group in the detailed metabolic and gene expression studies. RIS-AM dosing resulted in significantly higher liver weight (Fig. 1G) and increased levels of plasma triglycerides (Fig. 1H) compared with RIS-PM further suggesting that timing of dosing across the light/dark cycle significantly impacts metabolic side effects induced by APs.
A Food intake after risperidone (RIS) treatment, (Drug: F = 17.15, df = 1, p < 0.001, Time: F = 17.25, df = 1, p < 0.001, DrugXTime: F = 3.51, df = 1, p = 0.07) and B Food intake after OLZ treatment, (Drug: F = 24.79, df = 1, p < 0.001, Time: F = 13.22, df = 1, p < 0.001, DrugXTime: F = 5.35, df = 1, p = 0.03), C Weight gain after RIS treatment, (Drug: F = 27.6, df = 1, p < 0.001, Time: F = 30.35, df = 1, p < 0.001, DrugXTime: F = 21.72, df = 1, p < 0.001), D Weight gain after OLZ treatment (Drug: F = 5.27, df = 1, p = 0.03, Time: F = 10.86, df = 1, p = 0.003, DrugXTime: F = 3.27, df = 1, p = 0.07), E Fasting glucose (2 h post-treatment) of mice treated with (3 mg/kg) during the light (ZT2) or dark cycle (ZT11), (Drug: F = 0.11, df = 1, p = 0.74, Time: F = 27.41, df = 1, p < 0.001, DrugXTime: F = 26.19, df = 1, p < 0.001), F Fasting glucose (2 h post-treatment) of mice treated with olanzapine, (OLZ, 8 mg/kg) during the light (ZT2) or dark cycle (ZT11), (Drug: F = 2.85, df = 1, p = 0.1, Time: F = 3.00, df = 1, p = 0.09, DrugXTime: F = 1.47, df = 1, p = 0.24). A, C, E CON-AM n = 8, RIS-AM n = 7, CON-PM n = 8, RIS-PM n = 8. B, D, F CON-AM n = 5, OLZ-AM n = 7, CON-PM n = 6, OLZ-PM n = 6). G Liver weight (Drug: F = 21.08, df = 1, p < 0.001, Time: F = 56.58, df = 1, p < 0.001, DrugXTime: F = 1.5, df = 1, p = 0.23), H–L Plasma levels of triglycerides/hormones in mice treated with risperidone (3 mg/kg, CON-AM n = 6–8, RIS-AM n = 6–7, CON-PM n = 7–8, RIS-PM n = 6–8) during the light (ZT2) or dark cycle (ZT11). Data is expressed as mean ± SEM and analyzed using 2-Way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.05. Significance was set at p < 0.05. H Triglycerides (Drug: F = 11.68, df = 1, p = 0.24, Time: F = 42.7, df = 1, p = 0.0001, DrugXTime: F = 0.45 df = 1, p = 0.51), I Glucagon (Drug: F = 12.03, df = 1, p = 0.002, Time: F = 1.637, df = 1, p = 0.21, DrugXTime: F = 0.79, df = 1, p = 0.38), Secretin (Drug: F = 15.51, df = 1, p < 0.001, Time: F = 16.69, df = 1, p < 0.001, DrugXTime: F = 4.22, df = 1, p = 0.05), Pancreatic Polypeptide (PP, Drug: F = 3.29, df = 1, p = 0.08, Time: F = 4.46, df = 1, p = 0.04, DrugXTime: F = 0.17, df = 1, p = 0.68), Peptide YY (PYY, Drug: F = 1.35, df = 1, p = 0.25, Time: F = 2.07, df = 1, p = 0.16, DrugXTime: F = 2.98, df = 1, p = 0.09), J C-peptide (Drug: F = 14.78, df = 1, p < 0.001, Time: F = 2.56, df = 1, p = 0.12, DrugXTime: F = 1.23, df = 1, p = 0.27), Insulin (Drug: F = 2.33, df = 1, p = .13, Time: F = 0.30, df = 1, p = 0.58, DrugXTime: F = 0.04, df = 1, p = 0.83), Glucagon-Like Peptide 1 (GLP-1, Drug: F = 5.54, df = 1, p = 0.02, Time: F = 5.85, df = 1, p = 0.02, DrugXTime: F = 9.00, df = 1, p = 0.007), Gastric Inhibitory Peptide (GIP, Drug: F = 0.05, df = 1, p = 0.81, Time: F = 1.39, df=1, p = 0.24, DrugXTime: F = 11.22, df=1, p = 0.002), K Resitin (Drug: F = 5.81, df = 1, p = 0.02, Time: F = 0.08, df = 1, p = 0.78, DrugXTime: F = 0.44, df = 1, p = 0.51), Leptin (Drug: F = 21.08, df = 1, p < 0.001, Time: F = 56.58, df = 1, p < 0.001, DrugXTime: F = 1.5, df = 1, p = 0.23), L TNF-α (Drug: F = 2.23, df = 1, p = 0.14, Time: F = 1.39, df = 1, p < 0.24, DrugXTime: F = 1.50, df = 1, p = 0.23).
Many metabolic hormones are regulated by the circadian clock and rhythmically released. For this reason, we evaluated whether the changes in food intake and metabolism are associated with dysregulated hormone secretion. The significant effects of RIS on secretin, GLP-1 and GIP (Fig. 1I, J) are highly dependent on the time of dosing. RIS-AM decreased secretin to similar levels seen in CON-PM despite secretin levels being twice as high in CON-AM. RIS-AM treatment abolished the reduced GLP-1 levels seen between RIS-PM and CON-PM when compared to CON-AM (Fig. 1J). Interestingly, RIS-AM treatment reduced Gastric Inhibitory Polypeptide (GIP) GIP to levels comparable to CON-PM while RIS-PM increased GIP to CON-AM levels (Fig. 1J). Although RIS treatment in general decreased Glucagon (Fig. 1I) and C-peptide (Fig. 1J) the effect of RIS-AM is more evident than RIS-PM when compared to their respective vehicle controls. Leptin was increased during PM with no significant RIS effect (Fig. 1K). No significant effects of drug and time of dosing were observed with Pancreatic Polypeptide, Peptide YY (PYY), Insulin, Resistin and TNF-α (Fig. 1I–L). Therefore, overall, timing of AP dosing had significant effects on some metabolic hormone levels.
Time of dosing of APs significantly effects circadian gene expression
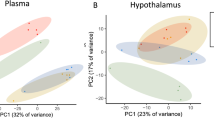
To examine the effects of APs on the expression of circadian genes, we evaluated gene expression at two time points (AM/PM) in the liver, hypothalamus and gonadal white adipose tissue (gWAT) of CON and RIS-treated mice. In the hypothalamus (Fig. 2A), consistent with the known patterns of variation across time, there is significant temporal differences on the gene expressions of many of these genes. For example, Per2 and Cry1 expressions were increased during the PM with Rev-erbα and Bmal1 expressions were lower at this time compared with CON-AM. The effects of RIS on hypothalamic Cry2 expression were time-dependent where RIS-PM increased Cry2 expression compared to its respective control. Peripheral tissues such as liver (Fig. 2B) and gWAT (Fig. 2C) demonstrated similar temporal profiles of circadian gene expression. In both tissues, the expression of Per1, Per2, Cry1 and Cry2 increased during the PM while Rev-erbα expression was lower at this time compared with CON-AM. Both tissues also responded similarly to time-dependent effect of RIS - with RIS-AM decreasing Rev-erba compared to CON-AM with no changes seen between CON-PM and RIS-PM. In both tissues, RIS-AM dosing decreased Clock expression compared with CON-AM control. Furthermore, RIS-PM resulted in significantly higher Clock expression than RIS-AM in both tissues. In addition, Per1 was also increased in RIS-AM in both tissues. However, there are some tissue differences observed. For example, RIS-AM significantly lowered liver expression levels of Cry2 and Rev-erbb compared with CON-AM but these genes were unchanged in the gWAT in RIS-AM versus CON-AM groups. In contrast, Clock, Per1 and Cry2 expressions were differentially expressed with RIS-PM compared wih CON-PM in the gWAT but were unchanged in the liver. In addition, gWAT Bmal1 expression was lower in RIS-PM compared to RIS-AM but unchanged in the liver (Fig. 2B, C). Overall, transcriptional analysis of the hypothalamus, liver and gWAT revealed RIS treatment induces significant changes in circadian gene expression in multiple tissues that is further perturbed by dosing in the AM. While the temporal resolution of these studies is limited, these time- and tissue depedent differences in circadian gene expression indicate that there may be widepread change in the circadian rhythm phase and/or amplitude caused by RIS in the gWAT and liver.
Circadian Gene Expression in A Hypothalamus, B Liver, C gWAT, of mice treated with risperidone (RIS, 3 mg/kg) or vehicle control (CON) during the light (AM = ZT2) or dark cycle (PM = ZT11) of mice treated with risperidone (RIS, 3 mg/kg) or vehicle control (CON) during the light (AM = ZT2) or dark cycle (PM = ZT11). Inflammatory gene expression in D Hypothalamus, E Liver, F gWAT, of mice treated with risperidone (RIS, 3 mg/kg) or vehicle control (CON) during the light (AM = ZT2) or dark cycle (PM = ZT11) of mice treated with risperidone (RIS, 3 mg/kg) or vehicle control (CON) during the light (AM = ZT2) or dark cycle (PM = ZT11). Data is expressed as mean ± SEM and analyzed using 2-Way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.05. Significance was set at p < 0.05, CON-AM n = 7–8, RIS-AM n = 6–7, CON-PM n = 7–8, RIS-PM n = 7–8). A Hypothalamus (Bmal Drug: F = 0.43, df = 1, p = 0.51, Time: F = 23.72, df = 1, p < 0.001, DrugXTime: F = 0.26, df = 1, p = 0.61, Clock Drug: F = 0.25, df = 1, p = 0.62, Time: F = 0.13, df = 1, p = 0.72, DrugXTime: F = 6.38, df = 1, p = 0.02, Per1 Drug: F = 2.19, df = 1, p = 0.15, Time: F = 8.36, df = 1, p = 0.007, DrugXTime: F = 0.29, df = 1, p = 0.59, Per2 Drug: F = 2.44, df = 1, p = 0.13, Time: F = 16.64, df = 1, p < 0.001, DrugXTime: F = 0.54, df = 1, p = 0.47, Cry1 Drug: F = 1.20, df = 1, p = 0.28, Time: F = 8.19, df = 1, p = 0.008, DrugXTime: F = 1.98, df = 1, p = 0.17, Cry2 Drug: F = 4.97 df = 1, p = 0.03, Time: F = 1.86, df = 1, p = 0.18, DrugXTime: F = 5.71, df = 1, p = 0.02, Rev-erba Drug: F < 0.01, df = 1, p = 0.99, Time: F = 18.63, df = 1, p < 0.001, DrugXTime: F = 2.16, df = 1, p = 0.15), B Liver (Bmal Drug: F = 0.07, df = 1, p = 0.78, Time: F = 0.24, df = 1, p = 0.62, DrugXTime: F < 0.01, df = 1, p = 0.98, Clock Drug: F = 4.1, df = 1, p = 0.05, Time: F = 112.1, df = 1, p < 0.001, DrugXTime: F = 6.38, df = 1, p = 0.02, Per1 Drug: F = 3.66, df = 1, p = 0.067, Time: F = 104.1, df = 1, p < 0.001, DrugXTime: F = 0.94, df = 1, p = 0.34, Per2 Drug: F = 26.32, df = 1, p < 0.001, Time: F = 532.7, df = 1, p < 0.001, DrugXTime: F = 13.38, df = 1, p < 0.001, Cry1 Drug: F = 0.013, df = 1, p = 0.91, Time: F = 61.82, df = 1, p < 0.001, DrugXTime: F = 0.28, df = 1, p = 0.60, Cry2 Drug: F = 3.40 df = 1, p = 0.07, Time: F = 37.47, df = 1, p < 0.001, DrugXTime: F = 0.86, df = 1, p = 0.36, Rev-erba Drug: F = 19.99, df = 1, p < 0.001, Time: F = 371.5, df = 1, p < 0.001, DrugXTime: F = 16.85, df = 1, p < 0.001, Rev-erbb Drug: F = 6.19, df = 1, p = 0.01, Time: F = 7.25, df = 1, p = 0.01, DrugXTime: F = 5.57, df = 1, p = 0.02), C gWAT (Bmal Drug: F = 0.15, df = 1, p = 0.70, Time: F = 28.25, df = 1, p < 0.001, DrugXTime: F = 0.81, df = 1, p = 0.37, Clock Drug: F = 8.60, df = 1, p = 0.008, Time: F = 0.072, df = 1, p = 0.79, DrugXTime: F = 2.47, df = 1, p = 0.13, Per1 Drug: F = 10.73, df = 1, p = 0.003, Time: F = 19.64, df = 1, p < 0.001, DrugXTime: F = 1.05, df = 1, p = 0.31, Per2 Drug: F = 16.15, df = 1, p < 0.001, Time: F = 155.9, df = 1, p < 0.001, DrugXTime: F = 10.35, df = 1, p = 0.03, Cry1 Drug: F = 0.06, df = 1, p = 0.80, Time: F = 23.71, df = 1, p < 0.001, DrugXTime: F < 0.01, df = 1, p = 0.97, Cry2 Drug: F = 8.20, df = 1, p = 0.008, Time: F = 12.11, df = 1, p = 0.002, DrugXTime: F = 0.32, df = 1, p = 0.57, Rev-erba Drug: F = 1.76, df = 1, p = 0.19, Time: 22.22, df = 1, p < 0.001, DrugXTime: F = 1.83, df = 1, p = 0.19, Rev-erbb Drug: F = 0.11, df = 1, p = 0.74, Time: F = 10.58, df = 1, p = 0.003, DrugXTime: F = 0.77, df = 1, p = 39), D Hypothalamus (Il1b Drug: F = 10.72, df = 1, p = 0.003, Time: F = 8.96, df = 1, p = 0.006, DrugXTime: F = 0.37, df = 1, p = 0.55, Il6 Drug: F = 1.31, df = 1, p = 0.26, Time: F = 0.37, df = 1, p = 0.54, DrugXTime: F = 0.32, df = 1, p = 0.57, Tnfa Drug: F = 0.14, df = 1, p = 0.70, Time: F = 1.89, df = 1, p = 0.18, DrugXTime: F = 7.16, df = 1, p = 0.013, Il10 Drug: F = 0.56, df = 1, p = 0.46, Time: F = 0.18, df = 1, p = 0.67, DrugXTime: F = 0.01, df = 1, p = 0.90, Cd11b Drug: F < 0.01, df = 1, p = 0.97, Time: F = 1.14, df = 1, p = 0.29, DrugXTime: F = 2.07, df = 1, p = 0.16, Cd11c Drug: F = 0.002, df = 1, p = 0.88, Time: F = 5.79, df = 1, p = 0.02, DrugXTime: F = 3.05, df = 1, p = 0.09), E Liver (Il1b Drug: F = 36.47, df = 1, p < 0.001, Time: F = 11.21, df = 1, p = 0.002, DrugXTime: F = 9.64, df = 1, p = 0.004, Il6 Drug: F = 0.18, df = 1, p = 0.67, Time: F = 0.19, df = 1, p = 0.66, DrugXTime: F = 1.20, df = 1, p = 0.28, Tnfa Drug: F = 0.05, df = 1, p = 0.81, Time: F = 16.2, df = 1, p < 0.001, DrugXTime: F = 0.56, df = 1, p = 0.45, Il10 Drug: F = 5.97, df = 1, p = 0.02, Time: F = 1.88, df = 1, p = 0.18, DrugXTime: F = 3.41, df = 1, p = 0.08, Cd11b Drug: F = 10.52, df = 1, p = 0.003, Time: F < 0.01, df = 1, p = 0.97, DrugXTime: F = 0.07, df = 1, p = 0.80, Cd11c Drug: F = 3.79, df = 1, p = 0.06, Time: F = 8.39, df = 1, p = 0.007, DrugXTime: F = 1.64, df = 1, p = 0.21), F gWAT (Il1b Drug: F = 1.67, df = 1, p = 0.20, Time: F = 1.76, df = 1, p = 0.19, DrugXTime: F = 6.15, df = 1, p = 0.02, Il6 Drug: F = 0.11, df = 1, p = 0.73, Time: F = 4.25, df = 1, p = 0.05, DrugXTime: F = 0.03, df = 1, p = 0.85, Tnfa Drug: F = 2.57, df = 1, p = 0.12, Time: F = 5.65, df = 1, p = 0.02, DrugXTime: F = 1.29, df = 1, p = 0.27, Il10 Drug: F = 0.11, df = 1, p = 0.74, Time: F = 11.05, df = 1, p = 0.003, DrugXTime: F = 13.39, df = 1, p = 0.001, Cd11b Drug: F = 2.15, df = 1, p = 0.15, Time: F = 0.07, df = 1, p = 0.80, DrugXTime: F = 1.67, df = 1, p = 0.21, Cd11c Drug: F = 0.20, df = 1, p = 0.65, Time: F = 1.57, df = 1, p = 0.22, DrugXTime: F = 4.0, df = 1, p = 0.06).
Time of dosing of APs significantly effects inflammatory gene expression
Our recent study identified that inflammation potentiates AP-induced hyperphagia and weight gain33. Since inflammation is regulated in part by the circadian clock34, we evaluated whether the timing of AP dosing affects inflammation in metabolically active tissues (hypothalamus, liver and gWAT). Hypothalamic expression of inflammatory markers did not change between CON-AM and CON-PM groups, but RIS AM dosing was associated with higher levels of proinflammatory genes including IL-1ß, IL-6, and TNFα compared with RIS-PM dosing (Fig. 2D). In the liver, the majority of genes were also unchanged by CON-AM vs CON-PM dosing, with the exception of TNF-α that was higher in CON-PM compared with CON-AM. TNF-α was also differentially expressed between RIS-AM and RIS-PM groups (Fig. 2E). Cd11b, a marker of myeloid cells was elevated in the liver by both RIS-AM/PM treatment groups compared with respective CON groups. Liver IL-1ß levels followed a similar pattern as observed in the hypothalamus whereby IL-1ß levels were elevated in both AP treatment groups compared with respective CON groups (Fig. 2E). Expression levels of Cd11c, a marker of proinflammatory macrophages, were elevated in the liver of RIS-AM treated mice compared with CON-AM but not different betweeen CON-PM and RIS-PM groups. Levels of IL-10, an anti-inflammatory cytokine were significantly lower in RIS-AM treated mice compared with RIS-PM. Inflammatory gene expression in gWAT was unchanged between CON-AM and CON-PM groups. RIS-AM treatment resulted in higher inflammatory tone in the gWAT compared with RIS-PM dosing. In particular, IL-1ß and IL-10 levels were increased in RIS-AM compared to RIS-PM (Fig. 2F). Therfore, RIS-AM dosing resulted in signifcant changes in inflamatory tone across multiple metabolic tissues including liver, gWAT and the hypothalamus.
TRF modulated the weight gain side-effect of RIS-AM treatment
Time restricted feeding (TRF) without calorie restriction is a therapeutic intervention against obesity and insulin resistance in both mouse and human studies16,35. Therefore, we tested whether TRF could mitigate RIS-induced metabolic impairments. Since the RIS-induced weight gain and impairments were most pronounced in the RIS-AM group, we used this dosing scheduling in TRF studies. As expected, RIS treatment in ad libiutm fed mice increased food intake (Fig. 3A), weight gain (Fig. 3B) and blood glucose levels (Fig. 3C) compared with CON treated ad libitum fed mice, Importantly, TRF significantly mitigated RIS-induced food intake (Fig. 3A), weight gain (Fig. 3B) and lowered blood glucose (Fig. 3C) compared with the ad libitum fed RIS treated group. Evaluation of expression of the core circadian genes revelaved key RIS-induced expression changes in liver Per1 was ‘rescued’ by TRF to equivalent CON expression levels (Fig. 3D). Furthermore, AP-induced inflammatory gene expression changes were also attenuated by TRF treatment. For example, Il-1ß levels were consistently elevated by RIS treatment compared with CON treatment in ad libitium fed mice, and TRF speficially reduced RIS-induced IL-1ß levels in hypothalamus, liver and adipose tissue compared with RIS-treated mice on the ad libitium diet. Other inflammatory markers, such as Il-6 and Tnf-α were also reversed by TRF in the hypothalamus (Fig. 4D).
A. Food intake (F = 12.21, df = 2, p = 0.004). B Body weight change (F = 8.25, df = 2, p = 0.004). C Blood glucose (2 h post-treatment, F = 10.78, df = 2, p = 0.001). D Circadian and Inflammatoy gene expression (Liver/Per1 F = 6.96, df = 2, p = 0.01, Hypo/Il1b F = 4.38, df = 2, p = 0.03, Liver/Il1b F = 10.05, df = 2, p = 0.001, gWAT/Il1b F = 7.86, df = 2, p = 0.004, Hypo/Il6 F = 4.90, df = 2, p = 0.02, Hypo/Tnfa F = 4.45, df = 2, p = 0.02) in metabolic tissues from mice treated with RIS during the light period with 24 h food access compared with mice treated with RIS with only 12 h food access. CON n = 6, RIS-AL n = 8, RIS-TRF n = 7. Data is expressed as mean ± SEM and analyzed using one-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.05. Significance was set at p < 0.05.
Changes in A Body weight (t = 2.413, df = 110.9, p = 0.02, day n = 102; night n = 2014), B HbA1c (t = 3.05, df = 19.61, p = 0.006, day n = 12; night n = 303), C Total cholesterol (t = 0.51 df = 20.08, p = 0.62, day n = 19; night n = 395), D Change in high-density lipoprotein (HDL, t = 1.67 df = 42.78, p = 0.10, day n = 19; night n = 395). E Change in low-density lipoprotein (LDL, t = 1.88 df = 20.91, p = 0.07, day n = 39; night n = 727). F Change in plasma triglycerides (t = 1.01 df = 21.41, p = 0.32, day n = 19; night n = 395), of patients taking risperidone (RIS) either during the daytime or nighttime using pharmacy records from the VA San Diego Healthcare System. Data is expressed as mean ± SEM and analyzed using Student T-test. Significance was set at p < 0.05.
Time of dosing impacts AP-induced metabolic side effects in humans
To examine the possibility of time-dependent AP effects in humans, we conducted a retrospective examination of weight and metabolic outcomes in patients who received RIS for the treatment of SMI using pharmacy records to estimate the time of RIS dosing. After controlling for treatment compliance, demographic and clinical factors, patients taking RIS-PM before the onset of sleep for ~1 year gained significantly more weight (Fig. 4A), had elevated glycosylated hemoglobin A1c (HbA1c) (Fig. 4B) compared to patients taking RIS-AM upon waking in the morning. These results are opposite to the differences observed in mice in terms of AM/PM, but similar to mice in terms of the relationship between AP dosing to sleep/activity schedules. No significant differences were identified in total plasma cholesterol, LDL, HDL and triglycerides (Fig. 4C–F).
Discussion
These studies investigated the impact of time of AP dosing on the development of weight gain and metabolic side effects. In nocturnal mice, AP dosing at the start of the less active light period (AM) resulted in significant increase in food intake, weight gain and metabolic side effects compared with equivalent dose just before the start of the active dark period (PM). Time of AP dosing also impacts circadian gene expression, metabolic hormones and inflammatory pathways and their diurnal expression patterns. We also observed an association between time of dosing and severity of RIS-induced metabolic side effects in patients with SMI prescribed APs. We demonstrate, for the first time, that TRF is an effective intervention to reduce weight gain effects of APs in mice.
Circadian rhythms influence feeding behavior, activity, and body weight with significant impacts on metabolism. Our studies revealed that AP-treatment impacted circadian gene expression across multiple metabolic tissues. Liver expression of Clock, Per1, Cry2 Rev-erbα and Rev-erbβ and gWAT expresison of Clock, Per1, and Rev-erbα were lower in mice dosed with RIS in the AM compared with vehicle control dosing at the same time. In support of the role of these genes impacting food intake, mice with whole-body deletion of Per1/2 or Cry1/236, or hypothalmic KO of Rev-erbα/Rev-erbβ37 eat more during the light period and less during the dark period compared with WT mice. Therefore, RIS induced decreases in these circadian genes during the inactive light period may contribute to the AP-induced hyperphagia seen in this group. The hypothalamic SCN clock is often resilient to perturbations by environmental inputs38 which may have limited the impact of APs on hypothalamic circadian gene expression in these studies. Furthermore, it is possible that time-dependent AP effects may be more apparent in individual hypothalamic nuclei, and are less pronounced when studying the whole hypothalamus.
Circadian regulation of hormones including insulin, glucagon, leptin and cortisol39,40 facilitate metabolic coordination and adaptation to periods of feeding and fasting and is a feature of optimal physiological function41. For example, insulin levels follow a regular circadian rhythm in humans, peaking during the day and dropping at night and disruption of molecular circadian rhythms causes insulin resistance and elevated blood glucose11,42. Prior studies have also shown that APs impact peripheral organs and dysregulate hormone regulation and metabolism43. Our data suggest RIS affects the physiological rhythms that coordinate metabolic hormones with feeding, fasting and activity behaviors in mice and humans. For example, RIS-AM treatment induced relative hyperglycemia compared with CON-AM or RIS-PM dosing. In addition, RIS-AM treatment also flattened the temporal profiles in blood levels of glucagon, GIP and secretin levels with losses of the diurnal peaks observed in CON mice. RIS-AM also significantly lowered C-peptide compared with vehicle CON and a similar trend of lower insulin in RIS-AM vs CON-AM was observed, which may contribute to the higher levels of glucose in RIS-AM vs CON-AM groups. Similar indications of sub-optimal metabolic function caused by RIS were shown by differences in inflammatory cytokines and changes in their diurnal profile, indicating the presence of a metabolic stress response particularly in the liver and to some extent hypothalamus, especially in the RIS-AM group. Taken together, these results indicate that, in additon to quantitative differences in hormone levels caused by RIS-AM, the drug also strongly affects the temporal coordination among hormone regulators of feeding behavior, blood glucose and metabolic activity depending on when the drug is administered. Furthermore, loss of temporal coordination among these regulators in RIS treated animals is associated with systemic inflammation and with more weight gain. The timing of our sampling limits our ability to distinguish phase and amplitude effects or to detect subtle differences in timing. Repeated sampling at intervals throughout the day will be important to described the effects of RIS on the oscillation of these genes over a 24 h cycle.
Importantly, the rest-activity cycle is reversed between rodents (nocturnal) and humans (diurnal). Our studies in both species align in that APs taken at the start of the rest period (AM in rodents, PM in humans) exacerbate the metabolic side effects compared with dosing at the start of the active period. Retrospective examination of pharmacy records in SMI patients who received RIS revealed that patients taking the medication at night gained significantly more weight and had elevated HbA1c compared to patients taking RIS in the morning. Importantly, these findings extend our published work reporting similarly unfavorable lipid profiles in SMI patients taking aripiprazole at night23. These data imply shifting the time of AP dose may be a clinical strategy to mitigate weight gain and metabolic side effects. However, on a practical level, APs also have a sedative effect that may pose challenges if taken at the start of the day. Thus, dosing at the start of the rest period while introducing time restricted eating could represent a highly effective alternative strategy that could be implemented to mitigate AP-induced side effects regardless of timing. When the consumption of high fat diet is restricted to an 8–9 h window during the active phase, TRF animals are protected from the adverse metabolic consequences, even though the total number of calories consumed was similar12,16. In our study, TRF in the 12 h active/dark phase abrogated RIS-induced weight gain and resulted in lower glucose levels compared with RIS treated ad libitum fed mice. TRF intervention in the RIS-AM group also resulted in 10% reduction in food intake which may have contributed to the reduced weight gain and overall improvements in glycemia. TRF was a highly effective intervention to reduce metabolic side effects in mice and it rescued AP-induced gene expression changes of key circadian and inflammatory genes in multiple metabolic tissues. For example, TRF restored liver expression of Per1 expression to equivalent level observed in the ad libitum control groups. With only two time points, our findings cannot determine whether TRF caused quantitative changes in circadian gene expression and/or normalized the phase of rhythmic expression profiles of circadian genes. In the present context, either outcome could be regarded as potentially beneficial and are in line with a role for TRF restoring rhythms in models of circadian disruption16. TRF also restored hypothalamic, liver and gWAT Il1ß gene expression to equivalent levels observed in the ad libitum control groups. Il1ß is a proinflammatory cytokine with well described roles in metabolic disease44 and neutralization of Il1ß improves glycemia and metabolic health in mouse studies45. Whether Il-1ß is causative of RIS-induced metabolic side-effects or consequence of increased body weight warrants further studies. Recent studies have shown that TRF has metabolic benefits even in animals that lack a circadian rhythm12. For example, when provided access to food ad libitum, whole-body Cry1/Cry2 and in liver-specific Bmal1 and Rev-erbα/ß knockout mice rapidly gained weight and showed genotype-specific metabolic defects. However, when fed the same diet under TRF (food access restricted to 10 h during the dark phase), they were protected from excessive weight gain and metabolic diseases. This suggests that the TRF is sufficient to properly entrain metabolic pathways that would be otherwise dysregulated by the lack or perturbed circadian rhythm or in psychiatric populations where internal clock may be compromised46,47. Time-restricted eating also mitigates weight gain and metabolic disease in humans35,48,49,50.
Our study has certain limitations. First, the animal model makes use of only female mice, while in humans weight gain and metabolic effects of AP are apparent both in males and females. Efforts are underway to develop AP-induced weight gain models in male mice to address this gap51. Next, APs have important effects on activity and sleep, but we do not have detailed information regarding these processes in mice that would help inform mechanisms related to weight gain. Future studies can make use of non-invasive sleep and activity monitors to determine if drug-induced changes in activity correlate with weight gain and the time-dependent effects of APs. Finally, our TRF protocol in mice did not have a control without APD treatment and allowed for a comparatively long feeding period (12 h) compared to other TRF animal studies (6–10 h). Additional work is required to identify the time windows that are most effective at mitigating weight gain and show the greatest potential for translation into human clinical populations receiving AP therapeutically. Although TRF studies in humans have varying effects on metabolic outcomes and further studies are needed to determine the most effective restriction protocols, TRF may still be a valuable alternative on how to mitigate AP-induced weight gain provided that the most effective TRF strategy is employed.
In summary, we provide evidence that strongly suggests that APs dosed at the wrong time in the circadian cycle perturb the temporal coordination of circadian and metabolic regulators to cause significant effects on weight gain and metabolic health in both mouse and human. In humans, retrospective pharmacy records-based data demonstrate worse effects of APs at night on weight and glycemia. While compelling, future randomized, prospective clinical studies are needed to fully examine the impact of timing on AP-induced metabolic health. TRF represents an exciting strategy to mitigate AP-induced metabolic side effects. However, patient compliance with TRF in the SMI population may be difficult as these patients often suffer from irregular sleep and activity patterns independently of AP treatment47. Moreover, APs can cause sleep disturbances52 with some patients reporting increased frequency of night eating after taking RIS53,54, OLZ55 and other APs56,57. In some patients, dosing APs before the rest period may perturb the circadian rhythm to drive aberrant feeding behavior. Therefore, clinical studies are necessary to test if TRF is an effective intervention to mitigate AP-induced metabolic side effects in patients.
Materials and methods
Mouse studies
All procedures were approved by the University of California San Diego IACUC. Female C57BL6/J mice were purchased from The Jackson Laboratory (Stock number: 000664, Sacramento, CA) at 9–10 weeks of age. Mice were acclimatized to the satellite housing conditions for 7 days and were housed in a room away from other studies to minimize any interruptions from other investigators/studies. Animals were maintained in a 12-h light:dark cycle with a humidity between 60 and 70%. At age 10–11 weeks mice were singly housed, provided unlimited access to water and normal chow food, and continued on a 12 h/12 h light dark schedule. Female mice were studied because they are particularly susceptible to AP-induced weight gain and reflect what is seen in both male and female SMI patients51.
Light and dark AP dosing
APs OLZ (8 mg/kg)6 or RIS (3 mg/kg)58 were self-administered to mice in a peanut butter/drug mixture pellet or vehicle control peanut butter alone31. This dose results in mouse plasma levels (21 ± 5 ng/ml) that are similar to levels observed in humans treated with OLZ (10–50 ng/mL)59. Mice were trained to eat peanut butter by fasting overnight followed by introduction of a peanut butter pellet (vehicle control, CON). Vehicle control pellets were then given to unfasted mice for an additional 3 consecutive days to overcome any novelty-associated behavior changes in feeding and locomotion. After training, mice consumed the pellet in less than ~15 min33 allowing for precisely timed AP dosing. Dosing time was ~2 h after lights on between 8:00–8.30AM, (Zeitgeber time (ZT) 2) for the ‘AM’ group and between 5:30–6:00PM, ZT 11 for the ‘PM’ group. No changes were made to the light/dark cycle. Dosing times were based on the start and end of the working day. At the end of the two-week study, mice were sacrificed 2 h after the last AP dose. AM groups were dosed at 8AM and sacrificed at 10AM while PM groups were dose at 6PM and were sacrificed at 8PM (n = 7–8 per group). Sample sizes were based on other similar studies6,31,33,51,60. Blood and tissues were dissected, flash frozen with liquid nitrogen and stored at −80 °C until analyses.
Time restricted feeding
Since the weight gain and metabolic effects of AP were only observed during the AM administration, we designed the TRF study using RIS-AM only. Mice were divided into 3 groups i) CON-AL: Fed normal chow ad libitum and treated with peanut butter control, ii) RIS-AL: Fed normal chow ad libitum and treated with risperidone in peanut butter, iii). RIS-TRF, restricted access to food and treated risperidone in peanut butter, n = 6–8 per group). We did not include a CON-TRF group as previous studies have investigated the impact of TRF on body weight and metabolism across a variety of dietary interventions12,16,61. Water was freely available at all times. In the CON-ad lib and RIS-AL groups, food was available 24 h a day. In the TRF group, food access was removed between ZT0 and ZT12 and restored during the active phase (ZT12–24) using specifically designed cages with a rotating wheel designed to have slots loaded with food accessible only at scheduled times. Food intake was measured daily at ZT0 and ZT12 and body weight measured at ZT0. After 10 days, mice were sacrificed 2 h after AP dosing (dosed at 8AM, sacrificed at 10AM).
RNA extraction, quantitative PCR and determination of metabolic hormone levels
Total RNA was extracted using Trizol (Invitrogen) and RNeasy Extraction Kit (Qiagen) as recommended by the manufacturer. RNA concentration and quality were assessed using Nanodrop. cDNA was synthesized from 500 ng of RNA using High-Capacity cDNA transcription kit (Thermo Fisher). qPCR was performed using StepOne Plus (Applied Biosciences). Gene expression was normalized to housekeeping genes 36B4 for the liver62, Atp5e for both gonadal and brown adipose tissues63 and Hprt1 for the hypothalamus64. These genes are stable reference genes suitable for circadian studies in different tissues and mouse strains62,65. Our data also confirm no difference in the expression of these housekeeping genes between AM and PM vehicle groups (Supplementary Fig. 1). Circulating blood levels of cytokines and gut-derived hormones were measured by multiplex ELISA (MMHE-44k-15, Millipore Sigma, Burlington, MA, USA).
Human retrospective analysis
The study was reviewed and approved by the VASDHS IRB. Using pharmacy records from the VA San Diego Healthcare System (VASDHS), we conducted a retrospective study of timing, weight gain, and long-term metabolic outcomes in SMI patients (age 18–75 years, mean age 48.2 y, 62% European ancestry, 89% male sex) taking RIS for ~1 year. Due to the retrospective nature of this chart review study, the IRB determined that informed consent was not necessary. The start date was defined as the first day that RIS was released to the patient and the end date was defined as the start date plus 365 days. Measures of body weight, HbA1c, serum glucose, fasting lipids were included if the first measure preceded initiation of RIS and the interval between the first and second measures was 9–15 months (1 year ±3 months). Patients were considered to have taken RIS in the morning if the instructions indicated “morning”, “daily”, “qam” or “qday” and were considered to have taken RIS at night when the instructions indicated “qhs” or “bedtime”. Compliance of >0.8 over 1 year was estimated by the frequency of on time refills. Data from January 1, 2002 to December 31, 2016 were included in the analysis. Those taking ultra-low dose RIS (≤0.25 mg), formulations of injectable RIS or RIS concurrent with another AP were excluded. Clinical indications for RIS included schizophrenia, schizoaffective disorder, unspecified psychosis, bipolar disorders, depressive disorders, and post-traumatic stress disorder. Patients with a diagnosis of Alzheimer’s disease, Parkinson’s disease, dementia or other neurocognitive disorder were excluded. Subjects with existing metabolic disorders prior to starting RISP including T2D, essential hypertension, and hypercholesterolemia were excluded. For each variable, data were analyzed using ANCOVA comparing the change in metabolic parameters from start date to end date in the AM vs. PM group, with age, sex, race, and dose of RISP as covariates. Smoking, alcohol and substance use history were not reliably recorded in the pharmacy database and were not considered. The research was reviewed and approved by the VASDHS IRB. Body weight data was available for n = 102 “morning”, n = 2014 “evening” dosing, A1c data was available for n = 42 “morning”, n = 303 “evening” dosing, LDL data was available for n = 39 “morning”, n = 727 “evening” dosing, HDL, TG, Total Cholesterol data was available for n = 19 “morning”, n = 395 “evening” dosing times.
Statistics
All statistical analyses (GraphPad Prism V5.03 San Diego, CA) defined significance as α < 0.05. Two group analyses were performed via a two-tailed t-test. Analyses of three or more conditions were performed using one-way ANOVA or two-way ANOVAs as indicated. ANOVA analyses were followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.05. Error bars indicate standard error of the mean (SEM).
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files.
References
Verhaegen, A. A. & Van Gaal, L. F. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J. Endocrinol. Investig. 40, 1165–1174 (2017).
Laursen, T. M., Munk-Olsen, T., Nordentoft, M. & Mortensen, P. B. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. J. Clin. Psychiatry 68, 899–907 (2007).
Mukherjee, S. et al. Understanding the effects of antipsychotics on appetite control. Front. Nutr. 8, 815456 (2021).
Goncalves, P., Araujo, J. R. & Martel, F. Antipsychotics-induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur. Neuropsychopharmacol. 25, 1–16 (2015).
Li, Y. et al. Berberine alleviates olanzapine-induced adipogenesis via the AMPKalpha-SREBP pathway in 3T3-L1 cells. Int. J. Mol. Sci 17, 1865 (2016).
Perez-Gomez, A. et al. A phenotypic Caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nat. Commun. 9, 5272 (2018).
Baptista, T. et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can. J. Psychiatry 51, 192–196 (2006).
Deng, C., Lian, J., Pai, N. & Huang, X. F. Reducing olanzapine-induced weight gain side effect by using betahistine: a study in the rat model. J. Psychopharmacol. 26, 1271–1279 (2012).
Mehta, V. S. & Ram, D. Efficacy of ranitidine in olanzapine-induced weight gain: a dose-response study. Early Interv. Psychiatry 10, 522–527 (2016).
Silverman, B. L. et al. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr. Res. 195, 245–251 (2018).
Stenvers, D. J., Scheer, F., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Chaix, A., Lin, T., Le, H. D., Chang, M. W. & Panda, S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metabol. 29, 303–319.e304 (2019).
Wang, J. B. et al. Timing of energy intake during the day is associated with the risk of obesity in adults. J. Hum. Nutr. Diet 27, 255–262 (2014).
Arble, D. M., Bass, J., Laposky, A. D., Vitaterna, M. H. & Turek, F. W. Circadian timing of food intake contributes to weight gain. Obesity 17, 2100–2102 (2009).
Duncan, M. J. et al. Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol. Behav. 167, 1–9 (2016).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabol. 15, 848–860 (2012).
Prasad, M. et al. A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: a pilot study. Nutrients 13, 2148 (2021).
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabol. 27, 1212–1221.e1213 (2018).
Pellegrini, M. et al. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 21, 17–33 (2020).
Romo-Nava, F. et al. Olanzapine-induced early cardiovascular effects are mediated by the biological clock and prevented by melatonin. J. Pineal. Res. 62, e12402 (2017).
Viyoch, J. et al. Effect of haloperidol on mPer1 gene expression in mouse suprachiasmatic nuclei. J. Biol. Chem. 280, 6309–6315 (2005).
Romo-Nava, F. et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 16, 410–421 (2014).
Chipchura, D. A., Freyberg, Z., Edwards, C., Leckband, S. G. & McCarthy, M. J. Does the time of drug administration alter the metabolic risk of aripiprazole? Front. Psychiatry 9, 494 (2018).
Kapse, S. et al. Effect of a dosing-time on quetiapine-induced acute hyperglycemia in mice. J. Pharmacol. Sci. 133, 139–145 (2017).
Shamshoum, H., Medak, K. D., McKie, G. L., Hahn, M. K. & Wright, D. C. Fasting or the short-term consumption of a ketogenic diet protects against antipsychotic-induced hyperglycaemia in mice. J. Physiol. 600, 2713–2728 (2022).
Wei, H. et al. Dopamine D2 receptor signaling modulates pancreatic beta cell circadian rhythms. Psychoneuroendocrinology 113, 104551 (2020).
Bak, M., Fransen, A., Janssen, J., van Os, J. & Drukker, M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One 9, e94112 (2014).
Komossa, K. et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 3, CD006654 (2010).
Komossa, K. et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 1, CD006626 (2011).
(AHRQ) AfHRaQ. ClinCalc DrugStats database version 21.1 (Medical Expenditure Panel Survey (MEPS), Rockville, MD, 2008–2018).
Zapata, R. C., Zhang, D., Chaudry, B., Osborn, O. Self-administration of drugs in mouse models of feeding and obesity. J. Visual. Exp. (2021).
Halfdanarson, O. et al. International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur. Neuropsychopharmacol. 27, 1064–1076 (2017).
Zapata, R. C. et al. Conserved immunomodulatory transcriptional networks underlie antipsychotic-induced weight gain. Transl. Psychiatry 11, 405 (2021).
Scheiermann, C., Gibbs, J., Ince, L. & Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 18, 423–437 (2018).
Wilkinson, M. J. et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metabol. 31, 92–104.e105 (2020).
Kettner, N. M. et al. Circadian dysfunction induces leptin resistance in mice. Cell Metabol. 22, 448–459 (2015).
Adlanmerini, M. et al. Hypothalamic REV-ERB nuclear receptors control diurnal food intake and leptin sensitivity in dietinduced obese mice. J. Clin. Invest. 131, e140424 (2021).
Brown, S. A., Zumbrunn, G., Fleury-Olela, F., Preitner, N. & Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 12, 1574–1583 (2002).
Petrenko, V. et al. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl Acad. Sci. USA 117, 2484–2495 (2020).
Petrenko, V. et al. Pancreatic alpha- and beta-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 31, 383–398 (2017).
Perelis, M. et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250 (2015).
Morris, C. J. et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl Acad. Sci. USA 112, E2225–2234 (2015).
Farino, Z. J. et al. New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion. Mol. Psychiatry 25, 2070–2085 (2020).
Febbraio, M. A. Role of interleukins in obesity: implications for metabolic disease. Trends Endocrinol. Metab. 25, 312–319 (2014).
Osborn, O. et al. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine 44, 141–148 (2008).
Barandas, R., Landgraf, D., McCarthy, M. J. & Welsh, D. K. Circadian clocks as modulators of metabolic comorbidity in psychiatric disorders. Curr. Psychiatry Rep. 17, 98 (2015).
Landgraf, D., McCarthy, M. J. & Welsh, D. K. Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr. Psychiatry Rep. 16, 483 (2014).
Gabel, K. et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging 4, 345–353 (2018).
Hutchison, A. T. et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity 27, 724–732 (2019).
Chow, L. S. et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity 28, 860–869 (2020).
Zapata, R. C. & Osborn, O. Susceptibility of male wild type mouse strains to antipsychotic-induced weight gain. Physiol. Behav. 220, 112859 (2020).
Cohrs, S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs 22, 939–962 (2008).
Gunes, S. & Camkurt, M. A. Sleep-related eating disorder associated with risperidone: an adolescent case. J. Clin. Psychopharmacol. 36, 286–288 (2016).
Lu, M. L. & Shen, W. W. Sleep-related eating disorder induced by risperidone. J. Clin. Psychiatry 65, 273–274 (2004).
Paquet, V., Strul, J., Servais, L., Pelc, I. & Fossion, P. Sleep-related eating disorder induced by olanzapine. J. Clin. Psychiatry 63, 597 (2002).
Horiguchi, J. et al. Nocturnal eating/drinking syndrome and neuroleptic-induced restless legs syndrome. Int. Clin. Psychopharmacol. 14, 33–36 (1999).
Kobayashi, N. & Takano, M. Aripiprazole-induced sleep-related eating disorder: a case report. J. Med. Case Rep. 12, 91 (2018).
Cope, M. B. et al. Antipsychotic drug-induced weight gain: development of an animal model. Int. J. Obes. 29, 607–614 (2005).
Morgan, A. P. et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9, e115225 (2014).
Zapata, R. C. et al. Metabolomic profiles associated with a mouse model of antipsychotic-induced food intake and weight gain. Sci. Rep. 10, 18581 (2020).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metabol. 20, 991–1005 (2014).
Kosir, R. et al. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol. Biol. 11, 60 (2010).
Almeida-Oliveira, F., Leandro, J. G. B., Ausina, P., Sola-Penna, M. & Majerowicz, D. Reference genes for quantitative PCR in the adipose tissue of mice with metabolic disease. Biomed. Pharmacother. 88, 948–955 (2017).
Li, B. et al. Identification of optimal reference genes for RT-qPCR in the rat hypothalamus and intestine for the study of obesity. Int. J. Obes. 38, 192–197 (2014).
Nakao, R., Okauchi, H., Hashimoto, C., Wada, N. & Oishi, K. Determination of reference genes that are independent of feeding rhythms for circadian studies of mouse metabolic tissues. Mol. Genet. Metab. 121, 190–197 (2017).
Acknowledgements
This study was supported through National Institutes of Health grant R01DK117872 (O.O.), Department of Veterans Affairs BX003431 (M.J.M.) and Larry L. Hillblom Foundation Postdoctoral Fellowship 2019-D-007-FEL (R.Z.).
Author information
Authors and Affiliations
Contributions
R.Z., O.O. and M.J.M. designed research; R.C.Z., A.S., D.Y., B.C. and A.L. performed experiments and analyzed data; M.J.M. performed the human study; R.C.Z., M.J.M. and O.O. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zapata, R.C., Silver, A., Yoon, D. et al. Antipsychotic-induced weight gain and metabolic effects show diurnal dependence and are reversible with time restricted feeding. Schizophr 8, 70 (2022). https://doi.org/10.1038/s41537-022-00276-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00276-2