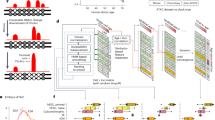
Abstract
The histone H3 variant H3.3, encoded by two genes H3-3A and H3-3B, can replace canonical isoforms H3.1 and H3.2. H3.3 is important in chromatin compaction, early embryonic development, and lineage commitment. The role of H3.3 in somatic cancers has been studied extensively, but its association with a congenital disorder has emerged just recently. Here we report eleven de novo missense variants and one de novo stop-loss variant in H3-3A (n = 6) and H3-3B (n = 6) from Baylor Genetics exome cohort (n = 11) and Matchmaker Exchange (n = 1), of which detailed phenotyping was conducted for 10 individuals (H3-3A = 4 and H3-3B = 6) that showed major phenotypes including global developmental delay, short stature, failure to thrive, dysmorphic facial features, structural brain abnormalities, hypotonia, and visual impairment. Three variant constructs (p.R129H, p.M121I, and p.I52N) showed significant decrease in protein expression, while one variant (p.R41C) accumulated at greater levels than wild-type control. One H3.3 variant construct (p.R129H) was found to have stronger interaction with the chaperone death domain-associated protein 6.
Similar content being viewed by others
Introduction
Histones are DNA-binding proteins constituting the building blocks of chromatin, i.e. the nucleosomes, and play an important role in the epigenetic regulation of chromatin compaction, gene transcription and other processes such as DNA damage repair. Each nucleosome is constructed from four core histones; H2A, H2B, H3, and H4, and a linker histone H1. Histone H3 consists of canonical isoforms H3.1 and H3.2, which can be replaced by multiple H3 variants such as H3.3 encoded by two genes H3-3A (also known as H3F3A) and H3-3B (also known as H3F3B) that are expressed in both embryonic and differentiated cells1,2,3.
The role of chromatin dysregulation in neurodevelopmental multisystemic disorders have been described by several studies4,5,6. Mutations in H3.3 genes have been implicated in human malignancies via promoting tumorigenesis by perturbing H3.3 chromatin-related epigenetic functions7. A female individual with global developmental delay, short stature, acquired microcephaly, hypotonia, hypoplasia of the corpus callosum and cerebellum, cortical visual impairment, atrial septal defect, and dysmorphic facial features was reported to have a de novo missense variant in H3-3A8. Recently, de novo missense variants in H3-3A and H3-3B have also been reported to be associated with neurodevelopmental delay and neurologic abnormalities in a cohort of 46 individuals8,9. Here, we report de novo missense and stop-loss variants in H3-3A (n = 6) and H3-3B (n = 6) in 12 individuals with neurodevelopmental disorders and multiple system abnormalities and a potentially perturbed interaction between one H3.3 mutant and the chaperone death domain-associated protein 6 (DAXX) using transiently transfected cell models.
Results
Molecular findings
All but one individuals were found to have de novo missense variants in evolutionarily highly conserved amino acids encoded by either H3-3A or H3-3B (Fig. 1), while the remaining individual (Individual 10) was found to have a de novo stop loss variant in H3-3B, which is predicted to result in incorporation of additional nine amino acids to the wild type protein (Table 1). All but one variants have not been observed in gnomAD (v2.1 and v3.0)10 and TOPMed (Freeze 8) databases11, while the c.386G>A (p.(R129H)) variant in H3-3A of Individual 4 was observed once in TOPMed Freeze 8. In silico prediction scores of the variants are in favor of pathogenicity (Table 1)12,13,14,15. Both genes encode a protein with identical polypeptide sequences forming five helices and eight beta strands that are involved in protein–protein interactions, and eight of the eleven missense variants are located in helices.
Clinical findings
Clinical findings of 10 individuals with de novo H3-3A or H3-3B variants are summarized in Table 2 and Supplementary Table 1. All individuals had global developmental delays with gross motor, fine motor, and speech delays and intellectual disability. Short stature with or without failure to thrive were reported in eight (80%) individuals. Hypotonia (n = 8, 80%), gait difficulties (n = 7, 70%), and microcephaly (n = 6, 60%) were the most commonly reported neurological abnormalities, and seizures were reported in four (40%) individuals. Neurobehavioral abnormalities such as happy demeanor, autism, and stereotypic movements were also reported in five (50%) individuals. Although there is no emerging facial gestalt yet, dysmorphic facial features such as facial asymmetry, midface hypoplasia, thin upper lips, open mouth appearance, prognathia or pointed chin, and minor extremity abnormalities such as small hands and feet with fingertip pads were reported in eight (80%) individuals (Fig. 2). Four of seven (57%) individuals with brain MRI performed were reported to have structural brain abnormalities ranging from diminished white matter and hypomyelination to cortical dysplasia and leukoencephalopathy. Ophthalmologic, musculoskeletal, and gastrointestinal system problems were also reported in all individuals, similar to generally reported in other neurodevelopmental disorders. Two individuals also had congenital hypothyroidism.
Functional characterization
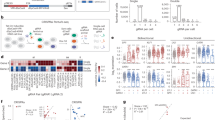
To determine the functional consequence of the H3-3A/H3-3B variants, we first attempted to determine protein levels of the 11 different mutants (one stop loss, ten missense) ascertained from the BG cohort in HEK293T cells. Mutagenesis was performed on cDNA clones in a pFLAG‐CMV‐4 expression vector using inverse PCR-based site-directed mutagenesis (see the “Methods” section). Each variant was incorporated into the corresponding gene (H3-3A or H3-3B) according to the variant–gene relationship in Table 1. Plasmids encoding FLAG-tagged wild-type (WT) and mutant H3.3s were transiently transfected into HEK293T cells. Anti-FLAG immunoblotting using whole-cell lysates of the transfected cells revealed a persistent lower protein level of three variants (p.R129H, p.M121I, and p.I52N). On the contrary, one variant (p.R41C) accumulated at greater levels than WT (Fig. 3a). Other variants did not show significantly altered protein levels. Notably, the c. 410_411del (p.*137Cext*9) variant in H3-3B resulted in an elongated protein with a similar protein level compared to WT (Fig. 3a). These results indicated differential impact of these variants on H3.3 protein abundance and stabilization in whole-cell lysates, while the exact mechanism for altered protein level is unclear.
a Levels of mutant histone H3.3s in HEK293T cells. FLAG-tagged WT and mutant histone H3.3s were transfected into HEK293T cells. Protein levels in whole-cell lysates were detected by immunoblotting with anti-FLAG antibodies. GAPDH was used as a loading control. The abundance of FLAG relative to GAPDH was estimated by densitometry with the ImageJ Software 1.52 v. Plot presents mean ± SD of immunoblotting analysis from three independent HEK293T cell transfections. Statistical analysis was performed by unpaired Student’s t test, with *p < 0.001. b Visualization of the crystal structural of H3.3-H4 heterodimer in complex with its chaperone DAXX. Shown on the left is a crystal structure of histone H3.3 based on template structure SWISS-Model P84243, with the position of the identified variants presented in this study. The variants are indicated by yellow spheres. Shown on the right is an overview structure of the DAXX–H3–H4 complex based on template structure PDB:4HGA, with variants found in affected individuals (black arrows). The H3.3 monomer, H4 monomer, and DAXX are colored in orange, purple, and green, respectively. Histone H3.3 variants (p.Q56, p.R129) directly interact with DAXX, likely affecting their interaction. c Co-immunoprecipitation of FLAG-tagged H3.3s with DAXX in HEK293T cells. A FLAG-tagged mutant H3.3 and a DAXX were co-transfected into HEK293T cells. Cells expressing FLAG-tagged WT H3.3 and DAXX were used as a control. Co-immunoprecipitation was performed with nuclear proteins. The samples were derived from the same experiment or parallel experiments and the gels/blots were processed in parallel. The abundance of DAXX relative to FLAG was estimated by densitometry with the ImageJ Software 1.52v. Plot presents mean ± SD of immunoblotting analysis from three independent HEK293T cell transfections. Statistical analysis was performed by unpaired Student’s t test, with *p < 0.05. WT wild type, GAPDH glyceraldehyde 3-phosphate dehydrogenase, SD standard deviation, DAXX death domain-associated protein 6.
Histone H3.3 interacts with histone H4 to form the H3.3–H4 heterodimer and cooperates with death domain-associated protein 6 (DAXX) to direct the deposition of H3.3 onto pericentric and telomeric heterochromatin16,17,18. Crystal structures of the DAXX–H3.3–H4 complexes (PDB:4HGA/4H9N/4H9O/4H9P/4H9Q) suggest that two residues (Q56 and R129) of H3.3 directly interact with DAXX by forming a hydrogen bond16,19. In addition, a previous study showed that the G91 in the ‘AAIG’ motif (A88, A89, I90 and G91) is a dominant contributor to chaperone specificity19. Therefore, the substitution of these residues with a different amino acid (in our cases p.Q56K, p.G91R and p.R129H) might affect protein–protein interaction with chaperones such as DAXX (Fig. 3b). To investigate this hypothesis, we co-transfected HEK293T cells with a FLAG-tagged mutant histone H3.3 (p.Q56K, p.G91R, and p.R129H, respectively) and a WT DAXX, and their association was analyzed by co-immunoprecipitation (Co-IP) of nuclear proteins. The Co-IP assays showed that p.R129H had a significantly stronger interaction with DAXX, suggesting that this variant might affect the deposition of histone H3.3 (Fig. 3c). To profile the intracellular distribution of histone H3.3 mutants, we next transfected FLAG-tagged mutants into HEK293T cells, and immunofluorescence was subsequently performed. The results revealed that both WT and mutants were found to be unaffected and localized inside the nucleus (Supplementary Figs. 1 and 2).
Since the N-terminal tail of the histone H3.3 is targeted for post-translational modifications (PTMs) to regulate protein activity, we further used various in silico prediction programs to explore the possible impact on PTMs imposed by mutations of H3.3 residues (Supplementary Methods). In silico predictions suggested that p.R9C, p.K10E, p.T23K, p.Q56K, and p.*137Cext*9 might alter the PTMs of histone H3.3, including phosphorylation, glycosylation, methylation, acetylation, and ubiquitination, while other mutants (p.G91R, p.M121I, p.P122L, p.R129H, p.A8V, and p.I52N) did not show apparent change on PTMs (Supplementary Table 2 and Supplementary Fig. 3). To facilitate understanding of various mechanisms of the reported variants, all effects of the variants from the experiments and in silico predictions are summarized in Supplementary Table 3.
Discussion
Genes involved in chromatin regulation and histone remodeling have been implicated in many neurodevelopmental syndromes with multisystemic involvement4,5,6. Although the role of deleterious variants in the core histone proteins in human cancers have been studied extensively, particularly gliomas, chondrosarcomas, and giant cell tumors of the bone20, their effect on human development were not fully understood. Recently, individuals with neurodevelopmental problems carrying predicted pathogenic germline variants in H3-3A and H3-3B have been reported in the literature as well as deposited in ClinVar8,21,22,23,24.
The clinical findings of individuals carrying de novo H3-3A and H3-3B variants in this study overlap with those reported before and with chromatinopathies in general25. In a large series of 46 individuals, Bryant et al. reported global developmental delay in the majority of their individuals along with hypotonia (67%), seizures (50%), short stature (33%), microcephaly (26%), and brain anomalies on imaging (73%)21. A moderate-to-severe global developmental delay was also observed in all individuals in our study, which usually affects all three major domains of psychomotor development, along with hypotonia (80%), gait abnormalities (70%), microcephaly (60%), variable structural brain abnormalities (57%), and seizures (40%). While short stature is reported in only one third of individuals reported by Bryant et al., all but two individuals in our study were reported to have short stature with or without failure to thrive. Furthermore, four individuals in our study also had endocrine system abnormalities including hypothyroidism, type 1 diabetes mellitus, advanced bone age, and precocious puberty. Bryant et al. also reported hypothyroidism in two of their individuals21, which altogether may suggest the endocrine system as being a major affected system. Craniosynostosis/abnormal head shape was reported by Bryant et al. in 30% of their individuals, and four (40%) in our study were reported to have possible craniosynostosis, facial asymmetries, and brachycephaly. Additionally, as noted by Bryant et al. although the majority of the individuals had variable dysmorphic facial features, there is no emerging facial gestalt common to all affected individuals. In contrast, while progressive neurologic disease and atrial septal defect were reported by Bryant et al. in ~1/5 of their individuals, only two in our study were reported to have non-structural congenital heart disease, and none was reported to have progressive neurologic disease. These differences might be explained by the variability in the ages of the individuals and in the duration of follow-up time as well as by the severity of the cardiac defect that could be overlooked without performing echocardiogram in all individuals. Furthermore, other genomic and environmental factors can also contribute to this variation as individuals with the same variants in both studies manifest variable phenotypes. For example, while individuals with the same p.G91R variant in H3-3A in both studies (n = 2) had highly overlapping clinical findings, individuals with p.P122L variant in H3-3A (n = 2) and individuals with p.R9C variant in H3-3B (n = 2) differ between each other in terms of short versus tall stature, failure to thrive versus obesity, and presence of endocrine abnormalities. Seven of thirteen H3-3A variants reported by Kaplanis et al. were also reported by Bryant et al. and our study, however, the clinical findings of those individuals were not reported in the Kaplanis et al. study to allow clinical comparison. Future natural history and functional studies may both outline the full clinical spectrum in the affected individuals and allow genotype–phenotype correlations by also objectively evaluating the severity of neurodevelopmental delays in each individual.
Fifty of fifty-two (96%) variants reported so far are missense variants, consistent with the low missense variation seen in the individuals cataloged in gnomAD v2.1 (missense Z-scores = 3.16 and 2.88 for H3-3A and H3-3B, respectively). The remaining two stop loss variants reported in this paper and by Bryant et al. are not expected to result in nonsense-mediated decay (NMD). Consistent with the speculation of escaping NMD, western blot analysis of the variant in our study demonstrated a mutant protein with larger size and comparable abundance to the WT control. Three missense variants (p.G91R, p.M121I, and p.P122L) as well as allelic variants (p.R129C and p.T23I) affecting the same residues as in p.R129H and p.T23K of our study were also reported by Bryant et al., who showed the H3.3 proteins carrying those missense variants resulted in increased proportion of H3.3 in H321. Furthermore, we performed a Co-IP assay and demonstrated that p.R129H variant significantly enhanced the binding of histone H3.3 to DAXX, which might disrupt heterochromatic modifications and lead to aberrant transcription26.
Alternatively, the whole histone protein can be swapped out for a histone variant, often highly similar in protein sequence but with a distinct functionality27. Although H3-3A and H3-3B contain introns as opposed to the canonical histone genes and are replication-independent for their expression, the encoded H3.3 protein sequence differs only by five amino acids from H3.1 and H3.2 in human cells. It was recently shown that there is an equilibrium between canonical (H3.1/H3.2) and non-canonical (H3.3) isoforms during embryonic development in mice via transcription-independent and replication-dependent manner28. Additionally, Strobino et al. reported that loss of histone H3.3 homolog HIS-72 in C. elegans results in DNA replication defects29. Thus, the disruption of the equilibrium in the ratio of subunit components of H3 in the developing embryo might result in differential epigenetic modifications and replication defects that might have adverse cellular and developmental outcomes.
Histone proteins are subject to numerous posttranslational modifications, which among other functions serve as binding locations for chaperone proteins and transcription factors promoting or repressing transcription depending on the type of modification and its exact location. H3.3 has been shown to have binary interactions with at least 10 proteins including DAXX, DNMT3A, ZMYND11, and SETD2. These interactions take place at different residues for each interaction, and different variants might disrupt interactions with certain proteins. However, the associated neurodevelopmental phenotype reported to-date is highly consistent among all variant carriers with variable expression of the phenotype to some extent. This suggests a global unifying underlying mechanism for all disrupting variants that warrants further investigation. The unique protein–protein interaction disruptions might explain the variability in the extent of the phenotype.
Interestingly, none of the individuals reported so far has had any type of cancer, although the variants previously implicated in somatic cancer development were reported by Bryant et al., and we showed enhanced binding of H3.3 protein composed of mutated H3-3A isoform to DAXX which was also implicated in molecular mechanism of H3-3A-mediated cancer development20. Separate mechanisms might be speculated to underlie the tumorigenesis and disruption of normal embryonic development in humans. For example, variants affecting the lysine residues directly (such as K9M, K27M, and K36M) or indirectly (such as G34R) have been shown to perturb the methylation of corresponding lysine residues in cancers (see Review by Lowe et al. 2019)20. On the other hand, cancer development is also uncommon in individuals with neurodevelopmental disorders carrying pathogenic germline variants in the methyl-/demethyltransferases such as EHMT1 (Kleefstra Syndrome 1, MIM:610253), KMT2A (Wiedemann–Steiner syndrome, MIM:605130), and KDM4B30 that regulate methylation of those lysine residues in H3 protein.
In summary, we report de novo missense and stop-loss variants in H3-3A and H3-3B in 12 unrelated individuals with overlapping neurodevelopmental phenotypes. In silico predictions suggest modified patterns of PTMs for the H3.3 proteins with mutations affecting the N-terminal tail. In vitro studies using transiently transfected cell line model suggest differentially altered protein level and/or stronger protein-protein interaction with chaperone DAXX resulted from the H3.3 variants reported in this study. Further studies are warranted to uncover the underlying molecular mechanism of H3.3 defects.
Methods
Ascertainment
Eleven individuals with de novo variants in H3-3A or H3-3B were identified by retrospective review of ~16,000 individuals referred for clinical exome sequencing (ES) at Baylor Genetics Laboratories (BG) between December 2011 and December 2019 with a range of indications including multiple congenital anomalies and neurodevelopmental disorders. All individuals involved in the study provided informed consents for clinical ES. Inclusion of anonymized individuals with minimum clinical information as part of an aggregated study based on retrospective review of data from BG clinical laboratories was approved by the Institutional Review Board (IRB) of Baylor College of Medicine (BCM, IRB# H-41191). The twelfth individual (Individual 6) was identified through Matchmaker Exchange, thus only clinical and molecular genetics data were presented in the study31. Among the twelve individuals, ten were subsequently recruited into a research study to provide case-level detailed clinical information. These individuals were enrolled by obtaining written informed consents approved by the IRB of BCM (IRB# H-22769). The authors affirm that human research participants provided informed consent for publication of images in Fig. 2.
ES analysis
ES analysis was performed at BG as previously described32. Samples were also concurrently analyzed by SNP arrays (Illumina HumanExome-12 or CoreExome-24 array) for quality control of the ES data, as well as for detecting large copy-number variants (CNVs) and regions of absence of heterozygosity (AOH)33,34. Homozygous/hemizygous deletions were also analyzed using an in-house developed pipeline based on exome read-depth analysis as previously described35. The ES-targeted regions cover >23,000 genes for capture design (VCRome by NimbleGen®), including both coding and untranslated region exons. The mean coverage of target bases was >100×, and >95% of target bases were covered at >20×32. PCR amplification and Sanger sequencing was performed to verify all candidate variants in the probands according to standard procedures. Of note, reanalysis of ES data for individuals who had their first ES analysis prior to January 2020 was performed as described recently36 to evaluate for the presence of other potentially causative variants. No other potential molecular diagnoses contributed by other loci were identified by the reanalyses.
Plasmid construction
A full-length cDNA clone of human H3-3A and H3-3B (GenBank: NM_002107.7 and NM_005324.5) was amplified using polymerase chain reaction (PCR). The PCR amplicons were cloned into the NotI and BglII sites of the pFLAG‐CMV‐4 expression vector (Sigma‐Aldrich). Inverse PCR‐based site‐directed mutagenesis was performed using KOD‐Plus‐Neo Kits (KOD-401, Toyobo) according to the manufacturer’s instructions. Mutated plasmid was used to transform E. coli clones and validated by Sanger sequencing. The primers used in mutagenesis are provided in Supplementary Table 4.
Transient transfection of HEK293T cells and immunoblotting analysis
A HEK293T cell line was a gift from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Cells were cultured in Dulbecco’s modified Eagle medium (C11995500BT, Thermo Fisher) containing 10% fetal bovine serum (10099141c, Thermo Fisher) and 1% penicillin–streptomycin (15140122, Thermo Fisher). Cells seeded at 1.5 × 106 cells/well in six-well plates were transfected with plasmids (5.0 ug) encoding WT or mutant N-terminal FLAG-tagged H3.3s using Lipofectamine 3000 Transfection Reagent (L3000015, Thermo Fisher). The efficiency was examined by transfecting cells with eGFP‐pFLAG‐CMV-H3.3 plasmid. After a 24-h transfection, whole cell protein was extracted using RIPA Lysis and Extraction Buffer (89900, Thermo Fisher). SDS–PAGE and immunoblotting analysis was performed by standard methods. Anti‐DDDDK tag antibody (1:1000, ab1162, Abcam), binds to FLAG® tag sequence, and anti-GAPDH monoclonal antibody (1:4500, K200057M, Solarbio) were used as the primary antibodies. Peroxidase AffiniPure goat anti-rabbit IgG (H + L) (1:20,000, 111-035-003, Jackson ImmunoResearch) and peroxidase AffiniPure goat anti-mouse IgG (H + L) (1:20000, 115-035-003, Jackson ImmunoResearch) were used as the secondary antibodies. Immunoblot images were analyzed using Image Lab Software 3.0 (BioRad). The abundance of FLAG relative to GAPDH was estimated by densitometry with the ImageJ Software 1.52v. All experiments were performed in triplicate.
Co-transfection of DAXX with histone H3.3 mutants and co-IP
Plasmids encoding a WT or mutant N-terminal FLAG-tagged H3.3 (15.0 μg) and a WT DAXX (15.0 μg) were transiently co-transfected into HEK293T cells cultured in 10-cm dishes using Lipofectamine 3000 transfection reagent (L3000015, Thermo Fisher). After a 48-h transfection, nuclear proteins were extracted using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (78833, Thermo Fisher) with Halt™ Protease Inhibitor Cocktail (87786, Thermo Fisher). Dynabeads Protein G (40 μl, 1003D, Thermo Fisher) was incubated with anti-DDDDK antibody (6.0 μg, M185-3L, MBL International), binds to FLAG® tag sequence, for 4 h at room temperature. The Dynabeads protein G–Ig complexes were then incubated in 0.2 M triethanolamine with 20 mM dimethylpyrimidine (DMP)–2HCl for 30 min at room temperature with end-over-end rotation. After the crosslinking, the beads were mixed with nuclear proteins and incubated overnight at 4 °C with end-over-end rotation, followed by three washes with lysis buffer. Bound target proteins were then eluted by heating the beads for 10 min at 70 °C in SDS loading buffer (NP0007, NP0009, Thermo Fisher) and subjected to Western blotting. Anti-DDDDK antibody (1:1000, 20543-1-AP, Proteintech), binds to FLAG® tag sequence, and anti-DAXX antibody (1:5000, ab32140, Abcam) were used as the primary antibodies. Goat Anti-Rabbit IgG (H + L) (1:5000, ZB-2301, ZSGB-BIO) was used as the secondary antibody. The abundance of DAXX relative to FLAG was estimated by densitometry with the ImageJ Software 1.52v.
Immunofluorescence
The HEK293T cells were implanted on glass slides in 24‐cell plates and transfected with plasmids encoding WT or mutant N-terminal FLAG-tagged H3.3s (0.5 μg) using Lipofectamine 3000 Transfection Reagent (L3000015, Thermo Fisher). After a 24-h transfection, the glass slides were rinsed with phosphate‐buffered saline (PBS) and fixed in 4% formaldehyde (P1110, SolarBio). The fixed cells were then rinsed with PBS four times and blocked with PBS containing 25% goat serum and 0.3% Triton™ X‐100 (Sigma‐Aldrich) for 1 h. Anti‐DDDDK tag antibody (P01L072; GENE-PROTEIN LINK), binds to FLAG® tag sequence, was used as the primary antibody. Goat anti‐rabbit IgG (H + L) cross‐adsorbed secondary antibody and Cyanine 3 (A10520; Thermo Fisher) were used as the secondary antibodies. Dyed slides were mounted using VECTASHIELD mounting medium with DAPI (H-1200-10, Vector Laboratories). Fluorescence was visualized using a confocal microscopy (Nikon Eclipse Ti2).
In silico prediction of PTMs of histone H3.3
PTM sites, including phosphorylation, glycosylation, methylation, acetylation, and ubiquitination, were predicted using KinasePhos 2.037, GPP38, GPS-MSP39, PAIL40, and NR-2L41, respectively. The full-length amino acid sequences of WT or mutant H3.3s were inputted into the website servers to predict modified sites using default settings. The PTM sites provided by the computational approaches were then located on the amino acid sequences.
Statistical analysis
The one-tailed unpaired Student’s t test was used for statistical analysis of immunoblotting results. Statistical analyses were performed with SPSS version 21.0 and GraphPad Prism version 8.0.2.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The main data supporting the findings of this study are available within the paper and its Supplementary information. Reasonable request of additional experimental results and/or materials is feasible and is conditioned to protect research participants’ privacy. Most materials and reagents used in this study are commercially available. Families enrolled in this study did not provide additional consent to share raw dataset for exome sequencing and SNP array in a public repository. The reported variants have been submitted to ClinVar under the accession numbers from SCV001759938 to SCV001759949.
References
Maze, I., Noh, K.-M., Soshnev, A. A. & Allis, C. D. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 15, 259–271 (2014).
Bano, D., Piazzesi, A., Salomoni, P. & Nicotera, P. The histone variant H3.3 claims its place in the crowded scene of epigenetics. Aging (Albany, NY) 9, 602–614 (2017).
Filipescu, D., Müller, S. & Almouzni, G. Histone H3 variants and their chaperones during development and disease: contributing to epigenetic control. Annu. Rev. Cell Dev. Biol. 30, 615–646 (2014).
Larizza, L. & Finelli, P. Developmental disorders with intellectual disability driven by chromatin dysregulation: clinical overlaps and molecular mechanisms. Clin. Genet. 95, 231–240 (2019).
Yuan, B. et al. Global transcriptional disturbances underlie Cornelia de Lange syndrome and related phenotypes. J. Clin. Investig. 125, 636–651 (2015).
Jakovcevski, M. & Akbarian, S. Epigenetic mechanisms in neurological disease. Nat. Med. 18, 1194–1204 (2012).
Yuen, B. T. K. & Knoepfler, P. S. Histone H3.3 mutations: a variant path to cancer. Cancer Cell 24, 567–574 (2013).
Maver, A., Čuturilo, G., Ruml, S. J. & Peterlin, B. Clinical next generation sequencing reveals an H3F3A gene as a new potential gene candidate for microcephaly associated with severe developmental delay, intellectual disability and growth retardation. Balk. J. Med. Genet. 22, 65–68 (2019).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Taliun, D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021).
Ioannidis, N. M. et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 99, 877–885 (2016).
Rentzsch, P., Witten, D., Cooper, G. M., Shendure, J. & Kircher, M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2019).
Samocha, K. E. et al. Regional missense constraint improves variant deleteriousness prediction. Preprint at bioRxiv https://doi.org/10.1101/148353 (2017).
Jagadeesh, K. A. et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat. Genet. 48, 1581–1586 (2016).
Liu, C.-P. et al. Structure of the variant histone H3.3–H4 heterodimer in complex with its chaperone DAXX. Nat. Struct. Mol. Biol. 19, 1287–1292 (2012).
Goldberg, A. D. et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691 (2010).
Drané, P., Ouararhni, K., Depaux, A., Shuaib, M. & Hamiche, A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 24, 1253–1265 (2010).
Elsässer, S. J. et al. DAXX envelops a histone H3.3–H4 dimer for H3.3-specific recognition. Nature 491, 560–565 (2012).
Lowe, B. R., Maxham, L. A., Hamey, J. J., Wilkins, M. R. & Partridge, J. F. Histone H3 mutations: an updated view of their role in chromatin deregulation and cancer. Cancers 11, 660 (2019).
Bryant, L. et al. Histone H3.3 beyond cancer: Germline mutations in Histone 3 Family 3A and 3B cause a previously unidentified neurodegenerative disorder in 46 patients. Sci. Adv. 6, eabc9207 (2020).
Farwell Hagman, K. D. et al. Candidate-gene criteria for clinical reporting: diagnostic exome sequencing identifies altered candidate genes among 8% of patients with undiagnosed diseases. Genet. Med. 19, 224–235 (2017).
Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature 542, 433–438 (2017).
Kaplanis, J. et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature 586, 757–762 (2020).
Fahrner, J. A. & Bjornsson, H. T. Mendelian disorders of the epigenetic machinery: postnatal malleability and therapeutic prospects. Hum. Mol. Genet. 28, R254–R264 (2019).
Voon, H. P. J. & Wong, L. H. New players in heterochromatin silencing: histone variant H3.3 and the ATRX/DAXX chaperone. Nucleic Acids Res. 44, 1496–1501 (2016).
Soshnev, A. A., Josefowicz, S. Z. & Allis, C. D. Greater than the sum of parts: complexity of the dynamic epigenome. Mol. Cell 62, 681–694 (2016).
Ishiuchi, T. et al. Reprogramming of the histone H3.3 landscape in the early mouse embryo. Nat. Struct. Mol. Biol. 28, 38–49 (2021).
Strobino, M., Wenda, J. M., Padayachy, L. & Steiner, F. A. Loss of histone H3.3 results in DNA replication defects and altered origin dynamics in C. elegans. Genome Res. https://doi.org/10.1101/gr.260794.120 (2020).
Duncan, A. R. et al. Heterozygous variants in KDM4B lead to global developmental delay and neuroanatomical defects. Am. J. Hum. Genet. 107, 1170–1177 (2020).
Sobreira, N. L. M. et al. Matchmaker exchange. Curr. Protoc. Hum. Genet. 95, 9.31.1–9.31.15 (2017).
Yang, Y. et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 369, 1502–1511 (2013).
Dharmadhikari, A. V. et al. Copy number variant and runs of homozygosity detection by microarrays enabled more precise molecular diagnoses in 11,020 clinical exome cases. Genome Med. 11, 30 (2019).
Yuan, B. et al. CNVs cause autosomal recessive genetic diseases with or without involvement of SNV/indels. Genet. Med. 22, 1633–1641 (2020).
Lalani, S. R. et al. Recurrent muscle weakness with rhabdomyolysis, metabolic crises, and cardiac arrhythmia due to bi-allelic TANGO2 mutations. Am. J. Hum. Genet. 98, 347–357 (2016).
Liu, P. et al. Reanalysis of clinical exome sequencing data. N. Engl. J. Med. 380, 2478–2480 (2019).
Wong, Y.-H. et al. KinasePhos 2.0: a web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 35, W588–94 (2007).
Hamby, S. E. & Hirst, J. D. Prediction of glycosylation sites using random forests. BMC Bioinforma. 9, 500 (2008).
Deng, W. et al. Computational prediction of methylation types of covalently modified lysine and arginine residues in proteins. Brief Bioinforma. 18, 647–658 (2017).
Li, A., Xue, Y., Jin, C., Wang, M. & Yao, X. Prediction of Nepsilon-acetylation on internal lysines implemented in Bayesian discriminant method. Biochem. Biophys. Res. Commun. 350, 818–824 (2006).
Wang, P., Xiao, X. & Chou, K.-C. NR-2L: a two-level predictor for identifying nuclear receptor subfamilies based on sequence-derived features. PLoS ONE 6, e23505 (2011).
Acknowledgements
We would like to thank families for participation in the study. Parts of this study were supported by the National Human Genome Research Institute/National Heart, Lung, and Blood Institute (NHGRI/NHLBI) grant UM1HG006542 to the BHCMG, the Beijing Natural Science Foundation (JQ20032 to N.W., 7191007 to Z.W.), the National Natural Science Foundation of China (81822030 and 82072391 to N.W., 81930068 and 81772299 to Z.W., 81772301 and 81972132 to G.Q.), Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program, CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-B-030 to T.J.Z.), Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2019PT320025). Data reprocessing and matchmaking for individual 6 were provided by the Broad Institute of MIT and Harvard Center for Mendelian Genomics (Broad CMG) and was funded by the National Human Genome Research Institute, the National Eye Institute, and the National Heart, Lung and Blood Institute grant UM1 HG008900 and in part by National Human Genome Research Institute grant R01 HG009141. J.L.E. was funded by the McLaughlin Centre (grant #MC-2012–13, #MC-2014-11-1 and MC-2017–12) and CIHR-Champions of Genetics: Building the Next Generation Grant (FRN: 135730).
Author information
Authors and Affiliations
Contributions
V.O., Z.C., N.W. and B.Y. conceived the project and drafted the manuscript. Z.C., N.W. and B.Y. designed the experiments. V.O., Z.C., L.V., S.P., J.R., L.Z., H.D., S.Z., G.Q., T.J.Z., Z.W., L.M., P.L., J.R.L., C.M.E., N.W. and B.Y. performed the experiments and analyzed the data. E.C., A.G., J.K., A.L., C.M., H.M.P., A.S., J.S., S.S., C.-T.F., C.L.G., J.L.E., K.M., S.M.-K., S.E.M., C.M., G.B.S., E.M.B.-K., R.G., N.G.-O. and D.A.S. provided patient care and relevant clinical information. All authors read, critically reviewed, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Baylor College of Medicine and Miraca Holdings Inc. have formed a joint venture with shared ownership and governance of BG, formerly the Baylor Miraca Genetics Laboratories (BMGL), which performs chromosomal microarray analysis and clinical exome sequencing. S.P., L.M., P.L., and C.M.E. are employees of BCM and derive support through a professional services agreement with BG. J.R.L. serves on the Scientific Advisory Board of the Regeneron Genetics Center and has stock ownership in 23andMe. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okur, V., Chen, Z., Vossaert, L. et al. De novo variants in H3-3A and H3-3B are associated with neurodevelopmental delay, dysmorphic features, and structural brain abnormalities. npj Genom. Med. 6, 104 (2021). https://doi.org/10.1038/s41525-021-00268-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41525-021-00268-8