Abstract
Background
The Royal College of Ophthalmologists has produced guidelines for screening for hydroxychloroquine retinopathy. New imaging modalities had suggested an increased prevalence of retinopathy compared with previous reports. The aim of this study is to identify the real-life prevalence of hydroxychloroquine retinopathy in patients attending Manchester Royal Eye Hospital screening service over a 2-year period using The RCOphth diagnostic criteria.
Methods
Data were collected prospectively from all patients attending Manchester Royal Eye Hospital hydroxychloroquine screening service over the audit period. Results of Humphrey visual field tests, spectral-domain optical coherence tomography and fundus autofluorescence were collected as well as data on dose, indication, duration of treatment, and additional risk factors. Cases were identified as having definite, possible, or no retinopathy based on the 2018 RCOphth criteria. The data are not publicly available due to information that could compromise research participant privacy and confidentiality but are available upon request from the corresponding author.
Results
910 patients attended for screening. 566 were identified as being at risk of retinopathy (543 had been on treatment >5 years, 10 had renal impairment, 12 were on doses of >5 mg/kg/day, and one was concurrently on tamoxifen). The prevalence of HCQ retinopathy was 10/910 (1.09%) of all those screened, and 1.76% of those at risk (10/566). Six patients of those deemed at risk were identified as having definite hydroxychloroquine retinopathy, while four had possible retinopathy.
Conclusions
Our results show a prevalence of retinopathy largely consistent with reports from regional audits yet reveal a far lower estimate compared to previously reported figures.
Similar content being viewed by others
Introduction
Whilst originally used as an antimalarial medication of the aminoquinoline family, Hydroxychloroquine (HCQ) was approved for use for treatment of autoimmune rheumatologic diseases as a disease modifying agent in the United States in 1955 [1]. According to the National Institute for Health and Care Excellence guidelines TA375, HCQ can be used in combination with other immunomodulatory therapies (IMT) before considering moving on to treatment with biological agents [2]. Unlike other IMT notorious for causing renal or hepatic dysfunction and requiring stringent blood monitoring, HCQ is very well tolerated and safe for use for the treatment of autoimmune diseases even during pregnancy, and hence, is still being prescribed increasingly in contemporary practice by medical specialists and immunologists [3].
Despite its safety profile, reports of ophthalmic toxicity from HCQ had emerged, where the eye was found to be affected by whorl-like corneal epithelial deposits; a condition referred to as cornea verticillata, or maculopathy relating possibly to the concentration of HCQ into the retinal cells with consequent atrophy of the outer retinal layers and photoreceptors loss [4,5,6,7,8,9]. The corneal changes are however innocuous, and usually reversible upon cessation of HCQ, unlike the more serious retinopathy which can potentially lead to permanent vision loss [8, 9]. Typical retinal toxicity is identified as outer retinal structural abnormalities including thinning and disruption of the outer nuclear layer and/or ellipsoid in the parafoveal area demonstrated on spectral domain optical coherence tomography (SD-OCT) and changes in fundus autofluorescence (FAF) [5, 6, 10, 11]. Functional deficits appear as ring-like or partial ring-like scotoma on 10-2 visual field testing or subnormal response on multifocal electroretinography (mfERG) [8, 12].
To mitigate irreversible vision loss attributed to HCQ intake whilst maintaining the availability of HCQ to patients needing immunosuppression, screening programmes were developed. The Royal College of Ophthalmologists (RCOphth) has recommended incorporation of retinal screening for HCQ toxicity into hospital eye services via virtual clinics, where all patients are referred for monitoring particularly after 5 years of therapy and are reviewed annually thereafter whilst on treatment [13, 14]. As data emerging over the years has described a wide range for the prevalence of retinopathy relating to HCQ intake, the aim of this study is to identify the real-life prevalence of HCQ retinopathy in patients attending the screening service at a tertiary ophthalmic hospital; the Manchester Royal Eye Hospital (MREH), over a 2-year period [9, 15,16,17]. This service evaluation audit has adopted the RCOphth 2018 guidelines which were in effect during the time the audit was undertaken [14].
Methods
Data were collected prospectively and continuously from all patients attending MREH for HCQ screening over the time period from April 2018 to March 2020. Demographic and clinical details studied included patient’s age, gender, indication for HCQ use, dose, duration of treatment, as well as data on renal function (estimated glomerular filtration rate (eGFR) in ml/min/1.73 m2), and concurrent use of Tamoxifen.
Patients were screened according to RCOphth guidelines and reviewed in dedicated virtual clinic sessions [14]. If HCQ retinopathy was suspected, they were then reviewed in face to face clinic. Patients attending for HCQ screening were divided into two dedicated pathways, Baseline (patients referred for baseline screening prior to or shortly after commencing) and Annual (patients who had taken hydroxychloroquine for 5 years or more) pathways. For Baseline pathway, patients underwent macular imaging using Topcon SD-OCT. For Annual pathway, patients had widefield FAF, macular SD-OCT using a Heidelberg Spectralis (Heidelberg engineering, UK) and 10-2 Humphrey visual field (HVF) testing. All images were reviewed by a single advanced ophthalmic science practitioner who specialises in ophthalmic imaging, and if need be, by a senior ophthalmologist in charge of HCQ screening at MREH at the time. If the visual field test was suspicious of HCQ retinopathy, the patient was recalled for another visit to repeat the test. Multifocal electroretinogram was performed, if necessary, based on the results of retinal imaging.
As per RCOphth guidelines, a diagnosis of no, definite, or possible HCQ toxicity was made [14]. Definite retinal toxicity is identified by the presence of consistent abnormalities on both functional tests and retinal imaging outlined previously, whilst abnormality in one or the other is described as possible retinopathy. All test results were conveyed via written correspondence to the referring clinician, as well as the patient and their GP. Patients who had been on HCQ for longer than 5 years were given appointments for follow up the following year as well as those identified as having risk factors for retinal toxicity. This included patients taking more than 5 mg/kg/day, those also taking Tamoxifen, and those with renal impairment (eGFR of <60 ml/min/1.73 m2).
Results
910 patients visited the HCQ screening service during the study period. 344 patients attended for baseline screening. 43 (12.5%) patients had just commenced the treatment. For those patients already on HCQ, the mean duration of treatment was 100.93 weeks (±77.76 weeks). All patients attending Baseline screening were discharged from the screening service and invited to attend annual screening after 5 years of HCQ therapy, apart from 14 patients who were advised to attend annual screening due to impaired renal function (3), high dosage of HCQ (10) and concurrent use of Tamoxifen (1). Two patients who were discharged from the service due to associated macular pathology precluding effective screening for HCQ toxicity.
A total of 566 patients attended for annual screening (duration of treatment >5 years in 543 patients, dose >5 mg/kg/day in 12, eGFR < 60 ml/min/1.73 m2 in ten cases, and concurrent use of Tamoxifen in one patient). Some patients had attended on more than one occasion for annual reviews or for repeat visual field testing, and information from the latest visits for these patients was reviewed. Most of the referrals were for patients attending the Kellgren Centre for Rheumatology at the Manchester Royal Infirmary and the Rheumatology department at Wythenshawe Hospital (46.3% and 20.7% of referrals respectively). Others included those from the dermatology departments at Salford Royal and Withington Community Hospitals as well as those made by primary care physicians.
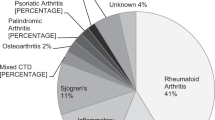
Of the 566 patients screened, 488 (86%) were female and 78 (14%) were male. Mean age was 56 years (±14 SD, range 16–91 years). Indications for treatment included rheumatoid arthritis (RA) in 200 patients (35.3%) and systemic lupus erythematosus (SLE) in 182 (32.2%). Other diagnoses included mixed connective tissue diseases and inflammatory arthropathies and are outlined in Table 1. The mean duration of treatment was 9 years (±5 years) for patients undergoing annual screening.
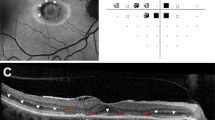
Visual field tests were available for all 566 patients. Of these, 508 (89.75%) were performed reliably, while 58 (10.25%) were classified as unreliable due to significant fixation losses, false positives and/or false negatives. 56 patients attended again for repeat HVF tests, while two patients failed to attend their appointments. Results from repeat HVF were described as normal in 52 patients while 3 were deemed as unreliable tests and were invited back for face to face consultation and 1 patient was invited back for repeat screening in 6-months’ time. Figures 1 and 2 show imaging and HVF findings in two cases from our series.
Patient was referred from the Rheumatology Unit with a history of SLE and has been using hydroxychloroquine at the dosage of 200 bd for 16 years. Fundus autofluorescence images (top) shows a semi-circle parafoveal ring of hyper-autofluorescence. OCT scans passing through the fovea (A1 and A2) show a subtle outer retinal change nasal to the fovea. Scans passing through the hyper-AF areas show significant outer retinal structural changes with thinning. Visual field (10-2) below shows areas of focal visual field loss in both eyes.
Patient was referred from the Rheumatology Unit with a history of SLE and has been using hydroxychloroquine at the dosage of 200 bd for more than 5 years. The patient had history of chronic renal disease with acute kidney injury and an estimated glomerular filtration rate of 37 mL/min/1.73m2. Fundus autofluorescence images (top) show a subtle increase in signal in parafoveal regions. OCT scans passing through the fovea (middle) show a subtle outer retinal change nasal to the fovea with an area of thinning in the left eye. Visual field (10-2) (bottom) shows areas of visual field loss in both eyes.
In this cohort, six patients have been diagnosed with definite HCQ retinopathy with abnormalities including perifoveal atrophic changes with loss of the ellipsoid zone on OCT and corresponding pericentral visual field defects. Two other patients were identified as having pathological changes possibly relating to HCQ toxicity where disruption in photoreceptor and outer retinal layers was identified alongside a normal visual field test (Repeat VF testing was unreliable in one of these patients, and following normal mfERG testing, the patient is currently being kept under close review in addition to reducing his HCQ dose from 400 to 200 mg/day). Two patients had field defects persistent on repeat testing in the presence of normal anatomy. The prevalence of definite HCQ toxicity using the 2018 RCOphth criteria in this patient population was 6/566 (1.06%), while that of possible retinopathy was 4/566 (0.7%) [14]. The overall prevalence of HCQ related retinopathy; both definite and possible, among our population at risk was 1.76%.
Discussion
HCQ sulphate is widely used for the treatment of autoimmune conditions [1, 18]. It is estimated that up to 51.7% of patients newly diagnosed with RA will be offered HCQ and 67% of SLE patients will commence HCQ within the first year [19]. It is understood its anti-inflammatory effects related to prevention of antigen processing and interruption of molecular pathways leading to pro-inflammatory cytokine release play a role in the prevention of autoimmune flare ups and could help prevent cytokine storms [1, 18, 20, 21].
However, in addition to cardiovascular morbidity and mortality associated with acute toxicity, long term use and high daily dosage may lead to irreversible and potentially blinding retinal toxicity; a risk further compounded by concurrent impairment of renal function or exposure to Tamoxifen [17, 22, 23]. The exact pathogenesis of this HCQ related retinopathy is not entirely clear, but as HCQ is of known affinity to melanin, it is proposed it will accumulate in melanin containing tissues of the body such as the eyes and skin causing cellular damage and atrophy through its effects on autophagosomes and lysosomes [1, 18]. In case of the retina, this damage occurs due to increased lysosomal pH with reduced phagocytic activity of RPE cells leading to accumulation of photoreceptor outer segments and retention of lipofuscin, which subsequently leads to RPE degeneration and photoreceptor loss [9, 17]. Some earlier studies however did suggest that the earliest changes occur rather in the inner retinal layers; namely ganglion cells and photoreceptors before evidence of RPE atrophy, suggesting that the sequence of events may still require further review [5,6,7].
Estimations of incidence of definite or possible retinal toxicity secondary to HCQ intake have varied widely in different studies [8, 9, 15, 24,25,26]. While some of the earlier studies recognised retinal toxicity following the development of significant central vision loss including the development of bull’s eye maculopathy, others have reverted to the use of diagnostic modalities such as central visual field tests and SD-OCT to demonstrate early retinal damage, suggesting an increased prevalence of retinopathy [8, 9, 17, 25]. Recent reports suggest a range between 1.58 and 10%, however there are wide variations in treatment duration and racial differences between the populations studied as well as variation in the screening recommendations used [4, 11, 13, 16, 24,25,26,27].
In view of the inherent risks, the RCOphth has recommended that all patients on HCQ should be referred for monitoring particularly after 5 years of drug intake and be reviewed annually thereafter whilst on therapy [14]. In 2018, the expert panel had recommended that patients should undergo 10-2 HVF testing and imaging with both SD-OCT and widefield FAF at each monitoring visit. Patients with reproducible significant visual field defects consistent with HCQ retinopathy, but without evidence of structural defects on SD-OCT or FAF were to be referred for mfERG. The college has also recommended that monitoring is commenced before 5 years in the presence of additional risk factors for toxicity including high dose of drug intake of more than 5 mg/kg/day, concomitant tamoxifen use, or renal insufficiency (eGFR of <60 ml/min/1.73 m2) [14].
A recommendation to stop HCQ should be made to the prescribing physician in case of definite toxicity; defined as abnormalities typical of HCQ retinopathy on two test results (one subjective test such as HVF and one objective test; SD-OCT or FAF). In case of possible toxicity (One test result typical of HCQ retinopathy, but typical abnormalities not present in other tests) patients should continue drug intake to reduce the chance of inappropriate treatment cessation whilst returning still for annual review. It is worth noting that these guidelines regarding protocols for annual monitoring have recently been updated following a review in December 2020, whereby only patients with abnormalities on either SD-OCT or widefield FAF would undergo visual field testing that is appropriate to the location of the abnormality on retinal imaging (10-2 visual field testing for patients with paracentral defects and 30-2 in case of pericentral disease) followed by mfERG if no abnormalities were identified on repeated visual field testing [28]. The panel have also concluded that baseline testing prior to commencing treatment was no longer recommended and that monitoring may be initiated after the first year in the presence of additional risk factors for retinal toxicity.
Our 2 years’ results for HCQ retinopathy screening at the MREH is largely consistent with a similar study from the UK where a prevalence of retinopathy of 1.2% was reported in a cohort of 333 patients at risk of retinopathy [16]. However, our data suggests a lower prevalence compared with the study by Marshall et al showing a prevalence of 1.6% for definite retinopathy and 4.7% for possible retinopathy in a group of 869 patients taking HCQ for more than 5 years and a far lower rate compared to results from similar reports in the literature [9, 15, 24, 29]. The incidence of retinopathy during the first 5 years of treatment is indeed rare, and in our cohort, none were identified as having HCQ related retinal pathology during the first 5 years of treatment [17]. The lower prevalence of retinopathy in our data may be multifactorial; possibly relating to shorter durations of treatment, different and heterogenous patient demographics, or differences in the risk characteristics with fewer patients in our group taking higher doses of HCQ. However, larger multicentre studies using standardised diagnostic criteria can provide accurate information on the prevalence of the condition and help further tailor screening efforts.
Summary
What was known before
-
HCQ is widely used for the treatment of autoimmune conditions, however, prolonged use may lead to irreversible and potentially blinding retinal toxicity.
-
A wide range for the prevalence of retinopathy relating to HCQ intake is described in the literature.
What this study adds
-
Results from this tertiary centre in the North West of England show a far lower prevalence of retinopathy compared to previously reported figures yet are largely consistent with reports from regional audits.
-
Real-life data is used by scientific and regulatory bodies to issue or review guidelines related to healthcare.
Data availability
The data are not publicly available due to information that could compromise research participant privacy and confidentiality but are available upon request from the corresponding author.
References
Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–53.
National Guideline Centre (UK). Rheumatoid arthritis in adults: diagnosis and management. London: National Institute for Health and Care Excellence (UK); 2018.
Beksac MS, Donmez HG. Impact of hydroxychloroquine on the gestational outcomes of pregnant women with immune system problems that necessitate the use of the drug. J Obstet Gynaecol Res. 2021;47:570–5.
Melles RB, Marmor MF. Pericentral Retinopathy and Racial Differences in Hydroxychloroquine Toxicity. Ophthalmology. 2015;122:110–6.
Pasadhika S, Fishman GA, Choi D, Shahidi M. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye. 2010;24:756–63.
Pasadhika S, Fishman GA. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye. 2010;24:340–6.
Ramsey MS, Fine BS. Chloroquine Toxicity in the Human Eye: Histopathologic Observations by Electron Microscopy. Am J Ophthalmol. 1972;73:229–35.
Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine. Ophthalmology. 2003;110:1321–6.
Melles RB, Marmor MF. The Risk of Toxic Retinopathy in Patients on Long-term Hydroxychloroquine Therapy. JAMA Ophthalmol. 2014;132:1453.
Lee DH, Melles RB, Joe SG, Lee JY, Kim J-G, Lee C-K, et al. Pericentral Hydroxychloroquine Retinopathy in Korean Patients. Ophthalmology. 2015;122:1252–6.
Ahn SJ, Joung J, Lim HW, Lee BR. Optical Coherence Tomography Protocols for Screening of Hydroxychloroquine Retinopathy in Asian Patients. Am J Ophthalmol. 2017;184:11–8.
Dettoraki M, Moschos MM. The role of multifocal electroretinography in the assessment of drug-induced retinopathy: a review of the literature. Ophthalmic Res. 2016;56:169–77.
Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016;123:1386–94.
Yusuf IH, Foot B, Galloway J, Ardern-Jones MR, Watson S-L, Yelf C, et al. The Royal College of Ophthalmologists recommendations on screening for hydroxychloroquine and chloroquine users in the United Kingdom: executive summary. Eye. 2018;32:1168–73.
Marshall E, Robertson M, Kam S, Penwarden A, Riga P, Davies N. Correction: prevalence of hydroxychloroquine retinopathy using 2018 Royal College of Ophthalmologists diagnostic criteria. Eye. 2021;35:358–358.
Gobbett A, Kotagiri A, Bracewell C, Smith J. Two years’ experience of screening for hydroxychloroquine retinopathy. Eye. 2021;35:1171–7.
Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye. 2017;31:828–45.
Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–66.
Ledingham J, Gullick N, Irving K, Gorodkin R, Aris M, Burke J, et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology. 2017;56:2257–2257.
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69.
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732–9.
Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res. 2010;62:775–84.
della Porta A, Bornstein K, Coye A, Montrief T, Long B, Parris MA. Acute chloroquine and hydroxychloroquine toxicity: a review for emergency clinicians. Am J Emerg Med. 2020;38:2209–17.
Eo D, Lee MG, Ham D-I, Kang SW, Lee J, Cha HS, et al. Frequency and Clinical Characteristics of Hydroxychloroquine Retinopathy in Korean Patients with Rheumatologic Diseases. J Korean Med Sci. 2017;32:522.
Manoj M, Sahoo RR, Singh A, Hazarika K, Bafna P, Kaur A, et al. Prevalence of hydroxychloroquine retinopathy with long-term use in a cohort of Indian patients with rheumatic diseases. Rheumatol Int. 2021;41:929–37.
al Adel F, Shoughy S, Tabbara K. Hydroxychloroquine dosing and toxicity: a real-world experience in Saudi Arabia of 63 patients. Saudi J Ophthalmol. 2020;34:151.
Fung AT, Lu V, Mack HG. Screening for hydroxychloroquine retinopathy in Australia. Med J Aust. 2021;215:434–434.
Yusuf IH, Foot B, Lotery AJ. The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): executive summary. Eye. 2021;35:1532–7.
Dadhaniya NV, Sood I, Patil A, Mallaiah U, Upadhyaya S, Handa R, et al. Screening for Hydroxychloroquine Retinal Toxicity in Indian Patients. J Clin Rheumatol. 2021;27:e395–e398.
Author information
Authors and Affiliations
Contributions
RA designed and registered the audit, interpreted the results, and drafted the paper. NB collected the data prospectively and revised and edited the paper. HS supervised the work, reviewed the data and study protocol, and revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alieldin, R.A., Boonarpha, N. & Saedon, H. Outcomes of screening for hydroxychloroquine retinopathy at the Manchester Royal Eye Hospital: 2 years’ audit. Eye 37, 1410–1415 (2023). https://doi.org/10.1038/s41433-022-02159-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02159-3
This article is cited by
-
Hydroxychloroquine
Reactions Weekly (2023)