Abstract
Introduction
To measure the prevalence of hydroxychloroquine retinopathy in patients attending a hydroxychloroquine monitoring service using 2018 Royal College of Ophthalmologists diagnostic criteria.
Methods
A service evaluation audit of a hydroxychloroquine retinopathy monitoring service was undertaken. Results of Humphrey 10–2 field tests, spectral-domain optical coherence tomography and fundus autofluorescence were collected with data on dose, weight, duration of treatment, estimated glomerular filtration rate, and concurrent tamoxifen therapy. Visual field tests were assessed as reliable or unreliable, and classified as normal, hydroxychloroquine-like, poor test or related to other pathology. Cases of definite and possible retinopathy were identified using the 2018 RCOphth criteria.
Results
There were 1976 attendances over two years of 1597 patients. Seven hundred and twenty-eight patients had taken hydroxychloroquine for less than 5 years and 869 had taken hydroxychloroquine for 5 years or more. Fourteen patients were identified with definite hydroxychloroquine retinopathy (1.6%), and 41 patients with possible retinopathy (4.7%). Sixty-seven per cent of 861 visual fields were performed reliably, with 66.9% classified as normal, 24.9% as poor test, 5.2% hydroxychloroquine-like and 3.0% abnormal due to other pathology.
Conclusions
The 1.6% prevalence of hydroxychloroquine retinopathy is lower than the previously reported prevalence of 7.5% as reported by Melles and Marmor JAMA Ophthalmol 132: 1453–60 (2014). This is because of a difference in the diagnostic criteria. Both definite and possible retinopathy would meet the diagnostic criteria of the Melles and Marmor study; 6.3% in our data, compared with 7.5%, a much smaller difference and likely to be explained by differences in the risk characteristics of the two groups.
Similar content being viewed by others
Introduction
Hydroxychloroquine (HCQ) is derived from quinine, an extract from the bark of the cinchona tree. Pharmacologically, it has multiple intracellular effects and is multimodal in its actions [1].
It is licensed in the UK for active systemic lupus erythematosus (SLE), discoid lupus, active rheumatoid arthritis and dermatological conditions aggravated by sunlight [2]. The use of hydroxychloroquine as an immunomodulatory agent is increasing, the number of patients taking the drug in 2016 was estimated at 166,673 with an additional 36,444 newly started in the same year [3]. Up to 67% of patients diagnosed with SLE start hydroxychloroquine within the first year as do 52% of those with newly diagnosed rheumatoid arthritis [4].
In SLE, hydroxychloroquine has been shown to have multiple benefits [5] including increased life expectancy [6], reduced risk of renal failure [7], protection against thrombotic events, diabetes mellitus, dyslipidaemia [8,9,10,11] and it has been shown to reduce arterial stiffness [12]. It has antibacterial and antiviral properties [13] and reduces infection rates in SLE [14].
Hydroxychloroquine can also be used in the treatment of antiphospholipid antibody syndrome [15], is safe in pregnancy [16], and may improve pregnancy outcome [17] in affected patients. A randomised controlled trial in pregnancy in mothers with antiphospholipid antibody syndrome is underway [18]. It may also be of benefit in ANCA-positive vasculitis [19] with a randomised clinical trial starting soon [20], in breast cancer [21] and other neoplasia [22]. There is in vitro evidence of effectiveness against COVID-19 viral infection [23].
Hydroxychloroquine has a better side effect profile than other disease-modifying agents and rarely causes renal and hepatic dysfunction, cardiotoxicity or skeletal muscle side effects at the appropriate (recommended) dosage.
The well-characterised retinal toxicity caused by hydroxychloroquine can lead to permanent vision loss. Studies on animals suggested that the earliest detectable change is in retinal ganglion cells and photoreceptors, with retinal pigment epithelium (RPE) changes occurring later [24, 25]. Hydroxychloroquine is concentrated into melanin-containing cells and reduces the internal pH of lysosomes, resulting in accumulation of lipofuscin [26], which may be partly responsible for the retinal toxicity.
A recent study of 2361 patients in USA suggested that the prevalence of toxicity overall was 7.5% when using central visual field (VF) testing or spectral-domain optical coherence tomography (SD-OCT) to detect early changes [27]. Hydroxychloroquine toxicity was identified as either anatomical abnormality (on SD-OCT or fundus autofluorescence (FAF)) or ring-like or partial ring-like scotoma on central 10–2 visual field assessment. Risk factors for toxicity were identified as dose >5 mg/kg actual body weight, duration, concurrent tamoxifen therapy and poor renal function.
In February 2018 the Royal College of Ophthalmologists (RCOphth) published new recommendations for the screening of patients taking hydroxychloroquine [28]. A diagnosis of definite retinal toxicity requires both function and anatomy to be abnormal, and abnormality in one test alone is classified as possible retinopathy.
St Thomas’ Hospital in London cares for a large number of patients being prescribed hydroxychloroquine by medical specialists including rheumatologists, dermatologists, and haematologists. A new service was started in October 2017 using draft guidance from the RCOphth and the formal guidance from Feb 2018 [28]. This paper discusses the findings in the first two years of the service.
Methods
The work was registered with the NHS Trust as a service evaluation audit. All patients referred were seen in an assessment clinic, staffed by a medical imaging specialist and ophthalmic technicians. Anonymised data with no personal identifiers were collected on indication, dose of drug, duration of treatment and current body weight. Patient age, gender, racial origin and estimated glomerular filtration rate (eGFR) were noted from the most recent result on the hospital electronic patient record system or calculated from the most recent creatinine level using a regression formula [29]. Data were collected for the time period 1st December 2017 to 30th November 2019 and analysed as per the 2018 guidelines [28]. A statistical comparison was made for the ratio data of age, duration, dose and eGFR. Tests of skewness and kurtosis were used to assess for normality and unpaired Student’s t test used for statistical comparison. Three tests were performed for each parameter (normal vs possible, possible vs definite, normal vs definite) and the Bonferroni correction indicates statistical significance should be set at p = 0.017.
Patients were separated into two groups, those who had been taking HCQ for less than 5 years and those taking for 5 years or more. All images and field tests were assessed for signs of hydroxychloroquine toxicity and for other pathology.
The 10–2 VF tests were noted as reliable or unreliable (based on the reliability indices) and the result graded as either normal or abnormal. Abnormal fields were subclassified into three groups ‘poor test’, ‘ring-like/partial ring-like’ or ‘other field pathology’. A test was classified as poor if there were scattered points of reduced sensitivity not in a ring-like distribution nor suggestive of other pathology or demonstrated artefact such as late starter or early finisher patterns. If the first visual field test was suspicious of hydroxychloroquine retinopathy the patient was recalled to repeat the test.
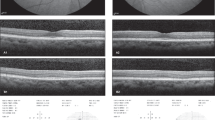
A diagnosis of definite HCQ toxicity was made if the patient had abnormalities of 10–2 visual field in a ring-like or partial ring-like scotoma accompanied by thinning/disruption of the outer nuclear layer and/or ellipsoid in the parafoveal area on SD OCT and changes on the blue autofluorescence images. Diagnosis was also made if the patient had repeated field tests suspicious of toxicity which was subsequently confirmed on multifocal electroretinography (mERG) testing.
Possible HCQ toxicity was diagnosed if the patient had an abnormal OCT or autofluorescence in the presence of a normal visual field test, or a ring-like or partial ring-like scotoma on the 10–2 field test which was persistent on a reliable second field test, in the presence of normal anatomical assessment.
Results
There were 1976 attendances in the 2-year period. Some patients attended on more than one occasion, either for annual review or for a repeat field test. To establish the correct denominator for prevalence calculation, the repeat visits were excluded and the information from the first visit used. The breakdown is shown in Table 1.
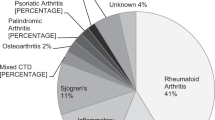
Seven hundred and twenty-eight patients had been taking hydroxychloroquine for less than 5 years, and 869 patients had been taking hydroxychloroquine for 5 years or more, a total of 1597 patients. Indications for treatment are shown in Table 2.
In the group taking HCQ for 5 years or more, 14 patients were identified as having definite toxicity. Twelve had abnormalities in both anatomy (OCT and FAF) and function; two were diagnosed after persistent field defects and assessment with mERG.
A total of 41 were diagnosed with possible retinopathy: 10 with abnormal anatomy on OCT (FAF was normal) and a normal visual field test; 31 had field defects persistent on second testing, in the presence of normal anatomy.
The prevalence of definite HCQ toxicity using the 2018 RCOphth criteria was 1.6%, of possible retinopathy was 4.7% and the overall prevalence of definite and possible retinopathy was 6.3%.
Data for age, duration of treatment, dose mg/kg and eGFR for patients taking HCQ for 5 years or more and the statistical comparison are shown in Table 3.
All tests of skewness were within +3 to −3. Kurtosis tests show values less than 3 except for duration in the possible group (κ = 4.0) and unpaired Student’s t test was used to calculate p values. Given the Bonferroni correction, the group with definite retinal toxicity were taking a statistically significantly higher dose (mg/kg) of hydroxychloroquine.
Visual field tests were available for 861 of 869 patients taking HCQ for more than 5 years. Of these 576 (66.9%) were performed reliably, the remaining 285 (33.1%) had fixation losses, false positives and/or false negatives sufficient to be classified as unreliable.
Five hundred and seventy-six (66.9%) were classified as normal, 214 (24.9%) were deemed poor tests, 26 (3.0%) had field defects related to pathology other than HCQ and 45 (5.2%) had HCQ-like field defects.
Discussion
In the first two years of the hydroxychloroquine service, we found a prevalence of definite toxicity of 1.6% and 4.7% possible toxicity with duration of treatment 5 years or more. The Melles and Marmor study [27] reported a prevalence of 7.5% for patients taking hydroxychloroquine for over 5 years. Their criteria used either a ring-like scotoma or SD OCT changes to diagnose retinopathy. This equates to the sum of both definite and possible retinopathy using the 2018 RCOphth criteria, which for these data is 6.3%. This much smaller difference is likely to be related to differences in the groups in terms of dose, duration of treatment and weight. Dose variation and compliance with the medication may also be important over a long period. The apparent larger difference between the observed prevalence of definite retinopathy and the US study is therefore due to the different diagnostic criteria used, rather than a difference in the clinical findings.
The analysis of dose shows a mean 3.9 (SD 1.5) mg/kg in the normal group and 5.3 (SD 1.6) mg/kg in the group with toxicity. The normal group had a shorter mean duration of 11.2 (SD 5.8) years taking hydroxychloroquine than the group with toxicity of 15.9 (SD 7.5) years. Although no initial power calculation was made and investigation of these measures was not a primary aim of the evaluation, there is a statistically significant difference in the dose (mg/kg) taken by the small group with retinal toxicity when compared with the normal group and close to statistical significance for the group with possible retinopathy compared with the normal; similarly for duration of treatment.
Dose and treatment duration are already established risk factors and the finding that the patients with toxicity were taking a higher dose of hydroxychloroquine for a longer duration than those without is not surprising; it indicates that the clinical assessment has detected a group of patients who were at higher risk of developing toxicity.
The results from the visual field tests showed ring-like scotomas in 5.2% of patients and this diagnosis was only made with reliable results and after a second field test. Three per cent (3%) of patients had reduced central visual fields related to other pathology, such as known glaucoma, previous retinal vein occlusion, macular scarring, previous optic neuritis and one patient had an asymptomatic bitemporal hemianopia which was related to a non-functioning pituitary adenoma demonstrated on an urgent magnetic resonance imaging (MRI) scan. Two patients had mERG performed because of suspicious field defects, and retinal dysfunction in the macular region was confirmed to be present.
The visual field test results showed a high proportion of unreliable and/or poor tests (33.1% and 24.9%, respectively). Repeat testing was needed on many occasions to identify whether the result was artefact or persistent. Visual field testing is a noisy investigation and the possibility of misinterpretation and incorrect classification exists.
Using the 2018 RCOphth criteria it is possible to obtain the same clinical outcome from the assessment if visual fields are performed only when the anatomical assessment is abnormal. If the OCT scan and FAF are normal in the absence of field testing, the patient has either no retinopathy or possible retinopathy, with the outcome being an annual review in both cases [26]. If the OCT/FAF is suggestive of hydroxychloroquine toxicity, a field test is needed to establish whether the patient has either possible or definite retinopathy. This methodology would result in the same number of diagnoses of definite retinopathy and would reduce the number of field tests needed considerably. This should help in reducing the noise in the investigation.
It seems sensible to suggest that the RCOphth guidance be revised to introduce a different methodology of delivering the monitoring service: Baseline visits would remain the same whilst those patients attending for review would have anatomical investigation with SD-OCT and FAF. If these tests suggest any degree of HCQ toxicity, the patient would perform a 10–2 field test. A diagnosis of definite HCQ toxicity would be made if the field test is reliable and demonstrates a true partial or full ring-like defect consistent with the anatomical disturbance. This change would facilitate hydroxychloroquine retinopathy monitoring that could be implemented across the nation without excessive burden on already-stretched services.
In conclusion, using 2018 RCOphth diagnostic criteria, we found a prevalence of 1.6% of definite retinopathy and 4.7% of possible retinopathy in a group of 869 patients taking hydroxychloroquine for more than 5 years. Prospective studies would be beneficial in establishing a more accurate prevalence measure.
Hydroxychloroquine is an excellent drug with significant benefits in autoimmune and other diseases due to its immunomodulatory, anti-inflammatory, anti-thrombotic and anti-proliferative effects. A diagnosis of hydroxychloroquine retinopathy should be made using robust measures as the consequence of treatment cessation is likely to be reactivation of systemic disease, with the need for alternative immunosuppression.
Summary
What was known before
-
Hydroxychloroquine is known to cause a maculopathy in a number of cases, dependent on dose, body mass and duration of treatment. Recent data from US suggests a prevalence of 7.5% when diagnosed using either central visual fields or SD-OCT. New RCOphth guidelines require both fields and SD-OCT to be abnormal to diagnose hydroxychloroquine retinopathy.
What this study adds
-
The prevalence of definite hydroxychloroquine retinopathy measured using RCOphth criteria is 1.6% The prevalence of possible retinopathy using RCOphth criteria is 4.7%.
Change history
20 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–53.
National Institute for Health and Care Excellence (NICE) 2020, Hydroxychloroquine sulfate, NICE, https://bnf.nice.org.uk/drug/hydroxychloroquine-sulfate.html.
Pearce FA, Grainge MJ, King AJ, Lanyon PC. Implementing screening for hydroxychloroquine ocular toxicity: how big is the problem? Epidemiology of hydroxychloroquine prescriptions in the UK clinical practice research datalink. Rheumatology. 2019;58:kez105.037.
Yates M, Malaiya R, Stack J, Galloway JB. Hydroxychloroquine use: the potential impact of new ocular screening guidelines. Eye. 2018;32:161–2.
Tang C, Godfrey T, Stawell R, Nikpour M. Hydroxychloroquine in lupus: emerging evidence supporting multiple beneficial effects. Intern Med J. 2012;42:968–78.
Alarcón GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alén J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007;66:1168–72.
Sisó A, Ramos-Casals M, Bové A, Brito-Zerón P, Soria N, Muñoz S, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus. 2008;17:281–8.
Costedoat-Chalumeau N, Dunogué B, Morel N, Le Guern V, Guettrot-Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med. 2014;43:e167–80.
Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus. 1996;5:S16–22.
Cairoli E, Rebella M, Danese N, Garra V, Borba EF. Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus. 2012;21:1178–82.
Chen YM, Lin CH, Lan TH, Chen HH, Chang SN, Chen YH, et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population-based cohort study. Rheumatol (Oxf). 2015;54:1244–9.
Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–82.
Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308.
Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67:1577–85.
Schreiber K, Breen K, Parmar K, Rand JH, Wu XX, Hunt BJ. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatol (Oxf) 2018;57:120–4.
Mekinian A, Lazzaroni MG, Kuzenko A, Alijotas-Reig J, Ruffatti A, Levy P, et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmun Rev. 2015;14:498–502.
Sciascia S, Hunt BJ, Talavera-Garcia E, Lliso G, Khamashta MA, Cuadrado MJ. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. 2016;214:273.e1–273e8.
Schreiber K, Breen K, Cohen H, Jacobsen S, Middeldorp S, Pavord S, et al. HYdroxychloroquine to improve pregnancy outcome in women with antiphospholipid antibodies (HYPATIA) protocol: a multinational randomized controlled trial of hydroxychloroquine versus placebo in addition to standard treatment in pregnant women with antiphospholipid syndrome or antibodies. Semin Thromb Hemost. 2017;43:562–71.
Casian A, Sangle SR, D’Cruz DP. New use for an old treatment: Hydroxychloroquine as a potential treatment for systemic vasculitis. Autoimmun Rev. 2018;17:660–4.
EU Clinical Trials Register: EU/EEA: 2020: EudraCT number 2018-001268-40. Hydroxychloroquine in ANCA vasculitis evaluation - a multicentre, randomised, double-blind, placebo-controlled trial; 2019. https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-001268-40/GB.
Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009;20:736–45.
Manic G, Obrist F, Kroemer G, Vitale I, Galluzzi L. Chloroquine and hydroxychloroquine for cancer therapy. Mol Cell Oncol. 2014;1:e29911.
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16.
Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978;17:1158–75.
Ding H, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatol (Oxf). 2016;55:957–67.
Sundelin S, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110:481–9.
Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453–60.
Imran H. Yusuf, Barny Foot, James Galloway, Michael R. Ardern-Jones, Sarah-Lucie Watson, Cathy Yelf, et al. Royal College of Ophthalmologists Guideline Development Group - Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Screening. https://www.rcophth.ac.uk/2018/03/rcophth-guideline-hydroxychloroquine-and-chloroquine-retinopathy-new-screening-recommendations-february-2018.
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marshall, E., Robertson, M., Kam, S. et al. Prevalence of hydroxychloroquine retinopathy using 2018 Royal College of Ophthalmologists diagnostic criteria. Eye 35, 343–348 (2021). https://doi.org/10.1038/s41433-020-1038-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1038-2
This article is cited by
-
A nationwide survey of hydroxychloroquine retinopathy presenting to the hospital eye service in the United Kingdom
Eye (2023)
-
Clock-hour topography and extent of outer retinal damage in hydroxychloroquine retinopathy
Scientific Reports (2022)
-
MERCI: a machine learning approach to identifying hydroxychloroquine retinopathy using mfERG
Documenta Ophthalmologica (2022)
-
Hydroxychloroquine screening in the UK-closing the gaps
Eye (2021)
-
The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): executive summary
Eye (2021)