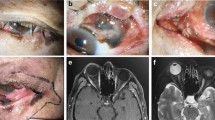
Abstract
Objectives
This study aimed to analyse the disease-free survival (DFS), overall survival (OS) and risk factors after orbital exenteration in patients with periorbital, conjunctival and primary intraorbital carcinomas.
Methods
Patients undergoing orbital exenteration due to a primary carcinoma between March 2000 and March 2018 were included in this retrospective study. Risk factors in all the patients were evaluated using univariate and multivariate analyses.
Results
In total, 97 patients were enroled in this study. The most common tumours were conjunctival carcinoma (35 cases), squamous cell carcinoma of the skin (27 cases) and basal cell carcinoma (20 cases). The median follow-up period was 36 months. The average age of the patients was 67.3 years (range, 29–93 years). In all the patients, OS was 85% after 1 year and 69% after 5 years, while DFS was 71% after 1 year and 55% after 5 years. Univariate analysis of OS revealed that the following parameters were predictive of a poor prognosis: localisation, neck dissection, lymph node metastases, lymphatic invasion, perineural invasion, resection margins and immunosuppression. Multivariate analysis revealed resection margins as the only independent risk factor.
Conclusion
Orbital exenteration is rarely necessary in patients with periorbital, conjunctival and primary intraorbital carcinomas; however, it can be performed as an ultima ratio treatment with a curative intent. Clear margins can be achieved in most cases. OS and DFS are not significantly different in the subgroups. In most cases, recurrence occurs within the first 2 years.
Similar content being viewed by others
Introduction
Basal cell carcinoma (BCC) is the most common malignant eyelid tumour among Caucasians, with an incidence of 90% [1], followed by squamous cell carcinoma (SCC), sebaceous cell carcinoma, Merkel cell carcinoma, melanoma and other rare malignancies [2]. Surgical excision is the gold standard treatment for periocular epithelial malignancies [3]. However, exenteration is necessary in a few cases.
Orbital tumours are less common than periorbital ones. Orbital tumours can be either intraconal or extraconal. Intraconal tumours are a heterogeneous group of malignant and benign neoplasms [4]. A cavernoma is the most common benign tumour, while sarcomas, lymphomas and metastatic lesions are the most common malignant tumours [5]. Extraconal tumours are mainly malignant tumours of the lacrimal gland [6]. Exenteration is particularly necessary in the case of malignant tumours if a resection with clear margins is not possible without sacrificing the eye or the optic nerve. As this radical operation is rarely necessary [7], there is little information on the survival and predictive factors after exenteration.
In this study, we aimed to evaluate the overall survival (OS) and disease-free survival (DFS) in patients with periorbital, conjunctival and primary intraorbital carcinomas after orbital exenteration. In addition, we analysed the prognostic factors in these patients.
Materials and methods
Patients
Patients undergoing orbital exenteration due to a carcinoma between March 2000 and March 2018 were identified and included in this retrospective study. Patients with enucleation as well as those who refused exenteration, which was recommended from a medical perspective, were excluded from the study. In addition, patients who were alive were excluded from the study if they had a follow-up period of <6 months. Patients with missing/incomplete records were also excluded. All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee (No. 18-8406-BO) and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was not obtained from the patients due to the retrospective study design. The data have been anonymised.
Orbital exenteration
The type of exenteration depended on the origin, localisation and extent of the underlying disease. Total (removal of all orbital contents), subtotal (preservation of some periocular tissue) or extended (additional removal of the bony orbit) exenteration was performed. All the cases were presented at an interdisciplinary tumour conference to determine further therapy before and after the operation.
Follow-up, survival and risk factors
The patients were followed up every month in the first year, every 2 months in the second year and every 6 months thereafter. OS was determined along with DFS, which was defined as the time from operation to relapse (local recurrence, lymph node metastasis or distant metastasis) or all-cause death, whichever occurred first. All carcinomas were divided into the following groups and subgroups: carcinomas (all), skin carcinomas, SCCs (all (skin and conjunctiva)), SCCs (conjunctiva), BCCs and carcinomas (others).
In addition, the following data were obtained: gender, site, localisation, recurrence of disease before exenteration, history of radiotherapy or chemotherapy before exenteration, vision loss or pain at presentation, type of exenteration, additional neck dissection (ND), reconstruction technique (granulation, eyelid adaptation, local flap reconstruction, microvascular grafting or split-skin grafting), time of reconstruction (none, primary, secondary), size or extent of the primary tumour (T), presence or absence of lymph node metastases (N) or distant metastases (M), tumour grade (differentiation) (G), resection margins (R0 = negative surgical margins or R1 = positive surgical margins), invasion into lymphatic vessels (L), perineural invasion, bone infiltration, number of resections, adjuvant therapies (radiotherapy and/or chemotherapy), history of immunosuppression, presence of a second tumour and relapse (local recurrence, lymph node metastasis or distant metastasis). The TNM classification used in this study is in accordance with the seventh edition of the TNM Classification of Malignant Tumours.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS Statistics version 21 (SPSS Inc.; IBM Company, Chicago, USA) and Microsoft Office 2010 Home and Student (Redmont, USA). Descriptive statistics were used to describe categorical patient characteristics. Kaplan–Meier plots were used to represent OS and DFS. The log-rank test was used to study differences. Multivariate analyses were performed using the Cox proportional hazards model. The level of statistical significance was set at p < 0.05 and p < 0.001.
Results
From March 2000 to March 2018, a total of 107 patients fulfilled the inclusion criteria. Three patients were excluded from the study because they had a follow-up period of <6 months, and six patients were excluded because of missing or incomplete records. In addition, one patient was excluded for refusing exenteration. Thus, a total of 97 patients (41 females and 56 males) were included in the study. The median follow-up period was 36 months. The average age of the patients was 67.3 years (range, 29–93 years). Sixty-seven patients (average age, 67.6 years) had SCC. Of these, 27 patients had SCC of the skin, 35 had SCC of the conjunctiva and 5 had SCC of the midface/sinus. Of the remaining patients, 20 had BCC, 3 had sebaceous gland carcinoma, 2 each had adenocarcinoma, adenoid cystic carcinoma and mucoepidermoid carcinoma and 1 had Merkel cell carcinoma. Thus, 47 patients (average age, 69.9 years) had epithelial skin carcinoma (27 SCC and 20 BCC). Of the total patients included in this study, the right side was affected in 55 patients, while the left side was affected in 42. Forty-four patients had a recurrent disease at the time of presentation.
The mean perioperative stay, including the staging, was 22 days. In 89 patients, a local R0 resection could be achieved through orbital exenteration. Of these, 14 patients needed more than 1 resection to obtain free margins. Adjuvant therapy was administered to 28 patients. Twenty-four patients (25%) had a relapse, of which nine patients had a local recurrence, fourteen had lymph node metastases and three had distant metastases. Of these, one patient had a local recurrence with a lymph node metastasis and another patient had a lymph node metastasis with distant metastases. Altogether, 26 patients with carcinoma died (27%) during follow-up. These included eight patients with SCC of the conjunctiva (23%), seven with SCC of the skin (26%), three with BCC (15%), three with sebaceous gland carcinoma (100%), two with adenocarcinoma (100%), two with SCC of the midface (40%) and one with Merkel cell carcinoma (100%). In all the patients, OS was 85% after 1 year and 69% after 5 years (Fig. 1a), while DFS was 71% after 1 year and 55% after 5 years (Fig. 1b). Table 1 provides a detailed overview of the patient characteristics, DFS and OS of the entire study population based on the studied parameters. Table 2 shows the results of univariate and multivariate analyses of risk factors associated with DFS and OS. Of the 47 patients with skin carcinoma, 53% had a recurrence at the time of presentation. ND was performed in 13 patients, 11 of whom had lymph node metastases. A local R0 resection could be achieved by orbital exenteration in 44 patients (94%). Bone infiltration was observed in 25 out of 26 patients who underwent extended exenteration. Sixteen patients had a relapse (34%). Seven of these patients had a local recurrence, eight had lymph node metastases and two had distant metastases. Of these, one had lymph node and distant metastases. Table 3 provides a detailed overview of the patient characteristics, DFS and OS with skin carcinoma based on the studied parameters.
Fourteen patients (30%) with skin carcinoma died during follow-up. In these patients, OS was 83% after 1 year and 56% after 5 years, while DFS was 67% after 1 year and 40% after 5 years. Specifically, in patients with SCC of the skin, OS was 78% after 1 year and 64% after 5 years, while DFS was 70% after 1 year and 47% after 5 years. In comparison, in patients with BCC, OS was 95% after 1 year and 80% after 5 years, while DFS was 79% after 1 year and 58% after 5 years.
In total, 35 patients had conjunctival SCC, and 29% of these had a recurrence at the time of presentation. An R0 resection could be achieved in most of these patients (34 patients). Six patients had a relapse (17%). Of these, one patient had a local recurrence and five had lymph node metastases. Table 4 provides a detailed overview of the patient characteristics, DFS and OS of patients with SCC of the conjunctiva based on the studied parameters (Table 4). In these patients, OS was 86% after 1 year and 82% after 5 years, while DFS was 73% after 1 year and 65% after 5 years.
Sixty-seven patients had SCC. Supplemental Table 1 provides a detailed overview of the patient characteristics, DFS and OS based on the studied parameters (Supplementary Material). In these patients, OS was 82% after 1 year and 75% after 5 years, while DFS was 73% after 1 year and 60% after 5 years.
Moreover, 15 patients had another carcinoma that could not be assigned to the aforementioned groups (including SCC of the midface). Seven of these patients had a relapse. Of these, one patient had a local recurrence, four had lymph node metastases and two had distant metastases. In total, eight patients died (53%). After 1 year, OS and DFS were 80% and 57%, respectively. OS and DFS were not calculated on the basis of the risk factors as the number of cases was small. The prognosis of patients with conjunctival carcinoma, BCC, SCC of the skin and other carcinomas did not differ significantly (OS, p = 0.117; DFS, p = 0.386; Fig. 1c, d).
Discussion
Compared with the existing literature, the present study has three major differences [1]: Orbital exenteration is rarely necessary due to improved diagnostic and reconstruction techniques. Thus, many existing studies include only small cohorts of less than 30 patients [8,9,10,11,12,13]. Only a few studies, such as the present study, have a comparable number of patients [2]. Of these, most studies consist of heterogeneous cohorts. Consequently, the number of orbital exenterations due to carcinoma differs in the literature. In Zhang’s study [14], 68% of the patients had carcinoma (69/102 cases). This number was 77% (61/79) in Hanasono’s study [15], 38% (38/100) in Kiratli’s study [16], 82% (31/38) in Kuo’s study [17], 70% (48/69) in Rahman’s study [18], and 61% (60/99) in Levin’s study [4]. Except for Rahman’s study, in all other studies (including ours), SCC was the largest subgroup. In contrast, only a few studies have included homogeneous cohorts. In the studies by Shields and Paridaens [19, 20], a total of 20 and 95 patients, respectively, had melanoma that led to orbital exenteration. In addition, Iuliano’s study included 28 patients with BCC [21], while Esmaeli’s study included 15 patients with adenoid cystic carcinoma [6, 3]. Many studies do not describe the difference between SCC of the skin and the conjunctiva. This is probably due to the rarity of conjunctival carcinoma, even though this carcinoma represents the largest subgroup in our cohort. Nevertheless, due to the rare necessity for exenteration, grouping heterogeneous cohorts is essential in order to elucidate risk factors and determine survival. The present study includes of one of the largest cohorts of patients with carcinomas reported so far. In addition, to our knowledge, no previous study has examined as many risk factors as examined in the present study.
The OS rates in the present study were similar to those in previous studies. OS was 88% after 1 year and 64% after 5 years in Wong’s study, 85% after 1 year and 72% after 5 years in Zhang’s study, 89% after 1 year and 57% after 5 years in Bartley’s study and 93% after 1 year and 57% after 5 years in Rahman’s study. [14, 22,23,24] Only one study reported a higher OS of 97% after 1 year and 84% after 5 years [16]. However, the cohort in that study was different, wherein retinoblastoma represented the largest subgroup (29%). In addition, 5% of the patients in the cohort had a benign disease. Moreover, the DFS rates in the present study were comparable with those in previous studies. Only a few previous studies have reported DFS after exenterations. In Kuo et al.’s study involving 38 patients, DFS was 83% after 1 year and 55% after 5 years [17]. Simons et al. and Bartley et al. found DFS to be 35% and 48%, respectively, after 5 years [22, 25]. In comparison, in the present study, DFS was 71% after 1 year and 55% after 5 years in all the patients. It should be noted that most relapses occurred within the first 2 years. BCC, SCC of the skin and conjunctiva accounted for 82 cases in our cohort. In total, there were 31 recurrences, only five (16%) of which occurred after the first 2 years. This finding was also apparent from the OS observed in the present study. Eighteen patients died in total; however, only two (11%) of these died after the first 2 years. In particular, in patients with conjunctival carcinoma, the first 2 years seem to be crucial for long-term survival. Of the 17 patients who survived the first year, only one died (6%). Thus, the survival after 2 and 5 years hardly differed in our cohort (data not shown). Or findings are in accordance with those reported previously [26].
Interestingly, the survival rates in previously reported heterogeneous cohorts were the same as those in our cohort, which consisted solely of patients with carcinoma. Moreover, the survival rates in our subgroups did not differ significantly. Despite the lack of statistical significance, there is a trend of BCC having the best prognosis, which seems attributable to the absence of distant metastases. In contrast, the subgroup carcinomas (others) in the present study tended to have the worst prognosis. Of the 15 patients in this subgroup, one had a local recurrence (7%), four had lymph node metastases (27%) and two had distant metastases (13%). In the present study, all the patients with sebaceous carcinoma and Merkel cell carcinoma died; these carcinomas have been associated with a poor prognosis in the literature [27, 28]. Moreover, we included patients with SCC of the midface in the present study, as it differs from SCC of the skin and conjunctiva due to the growth pattern and size. These tumours can infiltrate the cranial vault, nasal cavity, paranasal sinuses and maxilla, wherein the secondary participation of orbital contents is observed due to extensive expansion. Exenteration should only be performed as part of extensive resection if there is a significant survival benefit for the patient. As this is rare, we have only five cases in our cohort. These patients also had a poor prognosis, with OS of 62% after 1 year and 51% after 5 years [29].
The study of Gerring et al. included 49 patients, of which 22 had BCC, 17 had SCC and 10 had sebaceous gland carcinoma [26]. The median follow-up was 17.5 months, i.e. it was lower than that in our study. In that study, OS and DFS were 78% and 61%, respectively, after 2 years and 74% and 51%, respectively, after 5 years. Eight patients had recurrence after exenteration (16%). Univariate analysis of OS showed bone erosion, positive surgical margins and additional resection beyond exenteration as predictors of a poor prognosis, while multivariate analysis showed the presence of positive permanent margins as the only predictor of a poor prognosis. Interestingly, in the present study, multivariate analysis revealed resection margins as the only independent risk factor. This is more remarkable as a significantly larger number of risk factors were examined in the present study. However, this finding contradicts previously reported findings. Wong et al. reported that of the 73 patients in their study, 31 had clear surgical margins after exenteration and 42 did not have clear surgical margins [24]. They found no significant difference in life expectancy based on the surgical margin. The authors stated that the presence of clear surgical margins may prevent local recurrence but not micrometastases prior to surgery. Nevertheless, it should be noted that 27 of these patients had BCC and four had benign diseases; thus, no micrometastasis was expected in these cases, even though their surgical margins were not clear. Similar results were observed in Rahman’s study, in which 34 out of 64 patients had clear surgical margins, while 30 patients did not have clear surgical margins [23]. OS after 5 years was 57% in that study. There was no significant difference between the survival rates. Even in that study, BCC was the largest subgroup (44%).
Interestingly, in the present study, multivariate analysis revealed ND as an independent factor for recurrent disease in the entire cohort. ND was performed if there was a clinical and/or radiological suspicion of lymph node metastasis. A total of 17 out of 27 (63%) patients with ND actually had metastases, whereas only two out of 70 (3%) patients without primary ND later had lymph node metastasis. On the one hand, this could explain the recurrence due to the advanced disease stage at the time of exenteration. On the other hand, this aspect confirms the excellent diagnostic techniques that enable patient-specific therapies. Twenty-six patients with skin carcinoma underwent extended exenteration. Of these, 25 had bone infiltration (96%), which is also an evidence of improved diagnostic techniques.
In addition, it must be assumed that distant metastases lead to worsened OS. Orbital exenteration should only be performed if there is a significant survival-related benefit for the patient. Therefore, it is rarely necessary in patients with distant metastases. As only two patients had distant metastases in the present study, analysis was not possible given the small sample size; therefore, the factor had to be excluded. Exenteration was performed due to a blind and painful eye in these patients. One of these patients died after 3 months and the other died after 11 months.
Interestingly, the risk factor lymphatic invasion was not as crucial as we expected. In univariate analyses, this factor was found to be significant in most cases. In multivariate analyses, however, it was only found to be an independent factor in the SCCs (all, OS) group. Thus, the effect of lymphatic invasion in patients with carcinoma does not seem to be as important as that in patients with melanoma [30]. Larger (multicentre) studies are necessary to obtain more information.
In summary, orbital exenteration is rarely necessary in patients with periorbital, conjunctival and primary intraorbital carcinomas; however, it can be performed as an ultima ratio treatment with a curative intent. The following information should be considered when treating such patients [1]: Clear margins can be achieved in most cases [2]. The improvement in preoperative diagnostic techniques enables patient-specific therapy [3]. OS and DFS are not significantly different in these patient subgroups [4]. Most recurrences occur within the first 2 years [5]. Early detection of the primary disease is important for avoiding orbital exenteration.
Summary
What was known before
-
Orbital exenteration can be performed as an ultima ratio treatment with curative intent.
-
The improvement of preoperative diagnostics allows patient-specific therapy.
-
Risk factors after orbital exenteration remain unclear.
What this study adds
-
Clear margins can be achieved in most cases.
-
Most recurrences occur within the first 2 years.
-
Resection boundaries seem to be the only independent risk factor.
References
Shi Y, Jia R, Fan X. Ocular basal cell carcinoma: a brief literature review of clinical diagnosis and treatment. OncoTargets Ther. 2017;10:2483–9.
Silverman N, Shinder R. What’s new in eyelid tumors. Asia-Pac J Ophthalmol. 2017;6:143–52.
Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database, part I: periocular basal cell carcinoma experience over 7 years. Ophthalmology. 2004;111:624–30.
Levin PS, Dutton JJ. A 20-year series of orbital exenteration. Am J Ophthalmol. 1991;112:496–501.
Gunalp I, Gunduz K, Duruk K. Orbital exenteration: a review of 429 cases. Int Ophthalmol. 1995;19:177–84.
Esmaeli B, Ahmadi MA, Youssef A, Diba R, Amato M, Myers JN, et al. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2004;20:22–6.
Shields JA, Shields CL, Gunduz K, Cater J. Clinical features predictive of orbital exenteration for conjunctival melanoma. Ophthal Plast Reconstr Surg. 2000;16:173–8.
Croce A, Moretti A, D’Agostino L, Zingariello P. Orbital exenteration in elderly patients: personal experience. Acta Otorhinolaryngol Ital. 2008;28:193–9.
Goldberg RA, Kim JW, Shorr N. Orbital exenteration: results of an individualized approach. Ophthal Plast Reconstr Surg. 2003;19:229–36.
Kovacevic PT, Visnjic MM, Kovacevic TT, Radojkovic MR, Stojanovic MR. Extended orbital exenteration in the treatment of advanced periocular skin cancer with primary reconstruction with a galeacutaneous flap. Scand J Plast Reconstr Surg Hand Surg. 2009;43:325–9.
Rabey N, Abood A, Gillespie P, Athanassoglou V, Rene C, Malata CM. Reconstruction of complex orbital exenteration defects: a single center’s experience with a five-year follow-up. Ann Plast Surg. 2014;73:158–63.
Roche P, Timon C. Orbital exenteration in periorbital malignancies. Surgeon. 2012;10:189–93.
Taylor A, Roberts F, Kemp EG. Orbital exenteration-a retrospective study over an 11 year period analyzing all cases from a single unit. Orbit. 2006;25:185–93.
Zhang Z, Ho S, Yin V, Varas G, Rajak S, Dolman PJ, et al. Multicentred international review of orbital exenteration and reconstruction in oculoplastic and orbit practice. Br J Ophthalmol. 2017;0:1–5.
Hanasono MM, Lee JC, Yang JS, Skoracki RJ, Reece GP, Esmaeli B. An algorithmic approach to reconstructive surgery and prosthetic rehabilitation after orbital exenteration. Plast Reconstr Surg. 2009;123:98–105.
Kiratli H, Koc I. Orbital exenteration: institutional review of evolving trends in indications and rehabilitation techniques. Orbit. 2018;37:179–86.
Kuo CH, Gao K, Clifford A, Shannon K, Clark J. Orbital exenterations: an 18-year experience from a single head and neck unit. ANZ J Surg. 2011;81:326–30.
Rahman I, Cook AE, Leatherbarrow B. Orbital exenteration: a 13 year Manchester experience. Br J Ophthalmol. 2005;89:1335–40.
Paridaens AD, McCartney AC, Minassian DC, Hungerford JL. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520–8.
Shields JA, Shields CL, Demirci H, Honavar SG, Singh AD. Experience with eyelid-sparing orbital exenteration: the 2000 Tullos O. Coston Lecture. Ophthal Plast Reconstr Surg. 2001;17:355–61.
Iuliano A, Strianese D, Uccello G, Diplomatico A, Tebaldi S, Bonavolonta G. Risk factors for orbital exenteration in periocular Basal cell carcinoma. Am J Ophthalmol. 2012;153:238–41.e1.
Bartley GB, Garrity JA, Waller RR, Henderson JW, Ilstrup DM. Orbital exenteration at the Mayo Clinic. 1967-1986. Ophthalmology. 1989;96:468–73.
Rahman I, Maino A, Cook AE, Leatherbarrow B. Mortality following exenteration for malignant tumours of the orbit. Br J Ophthalmol. 2005;89:1445–8.
Wong JC, Thampy R, Cook A. Life expectancy following orbital exenteration. Br J Ophthalmol. 2015;99:1–4.
Simons JN, Robinson DW, Masters FW. Malignant tumors of the orbit and periorbital structures treated by exenteration. Plast Reconstr Surg. 1966;37:100–4.
Gerring RC, Ott CT, Curry JM, Sargi ZB, Wester ST. Orbital exenteration for advanced periorbital non-melanoma skin cancer: prognostic factors and survival. Eye. 2017;31:379–88.
Kyllo RL, Brady KL, Hurst EA. Sebaceous carcinoma: review of the literature. Dermatologic surgery: official publication for American Society for. Dermatologic Surg. 2015;41:1–15.
Schadendorf D, Lebbe C, Zur Hausen A, Avril MF, Hariharan S, Bharmal M, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69.
Iannetti G, Valentini V, Rinna C, Ventucci E, Marianetti TM. Ethmoido-orbital tumors: our experience. J Craniofac Surg. 2005;16:1085–91.
Paridaens AD, Minassian DC, McCartney AC, Hungerford JL. Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases. Br J Ophthalmol. 1994;78:252–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Baum, S.H., Pförtner, R., Manthey, A. et al. Periorbital, conjunctival and primary intraorbital carcinomas: Survival and risk factors after orbital exenteration. Eye 35, 1365–1376 (2021). https://doi.org/10.1038/s41433-020-1055-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1055-1