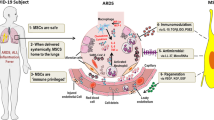
Abstract
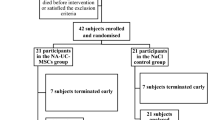
Mesenchymal stromal cells (MSC) have immunomodulatory and tissue-regenerative properties and have shown promising results in acute respiratory distress syndrome (ARDS) of multiple causes, including COVID-19. We conducted a randomised (1:1), placebo-controlled, double-blind clinical trial to assess the efficacy and safety of one bone marrow-derived MSC infusion in twenty patients with moderate to severe ARDS caused by COVID-19. The primary endpoint (increase in PaO2/FiO2 ratio from baseline to day 7, MSC 83.3 versus placebo 57.6) was not statistically significant, although a clinical improvement at day 7 in the WHO scale was observed in MSC patients (5, 50% vs 0, 0%, p = 0.033). Median time to discontinuation of supplemental oxygen was also shorter in the experimental arm (14 versus 23 days, p = 0.007), resulting in a shorter hospital stay (17.5 versus 28 days, p = 0.042). No significant differences were observed for other efficacy or safety secondary endpoints. No infusion or treatment-related serious adverse events occurred during the one-year follow-up. This study did not meet the primary endpoint of PaO2/FiO2 increase by day 7, although it suggests that MSC are safe in COVID-19 ARDS and may accelerate patients’ clinical recovery and hospital discharge. Larger studies are warranted to elucidate their role in ARDS and other inflammatory lung disorders.
Trial Registration: EudraCT Number: 2020-002193-27, registered on July 14th, 2020, https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002193-27/ES. NCT number: NCT04615429, registered on November 4th, 2020, https://clinicaltrials.gov/ct2/show/NCT04615429.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The de-identified participant data will be available to other researchers, for scientific purposes, upon request to the corresponding author, with approval from the COVID-AT Steering Committee.
References
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–9.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Della CG. et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750–2.
Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–60.
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704.
Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science (80-). 2020;369:eabc8511.
Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–43.
Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:1–16.
Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transpl. 2016;25:829–48.
Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells Mesenchymal Stem/Stromal Cells - An update. Stem Cell Res Ther. 2016;7:1–12.
Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–62.
Chen J, Hu C, Chen LL, Tang L, Zhu Y, Xu X, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering. 2020;6:1153–61.
Kirkham AM, Bailey AJM, Monaghan M, Shorr R, Lalu MM, Fergusson DA, et al. Updated living systematic review and meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a framework for accelerated synthesis of trial evidence for rapid approval-FASTER approval. Stem Cells Transl Med. 2022;11:675–87.
Payares-Herrera C, Martínez-Muñoz ME, Vallhonrat IL, de Molina RM, Torres MP, Trisan A, et al. Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:20–2.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: The Berlin definition. J Am Med Assoc. 2012;307:2526–33.
Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, Rico MA, Bautista G, Krsnik I, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy. 2009;11:278–88.
Fernández-Maqueda C, Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, Sánchez R, Bueno JL, et al. Mesenchymal stromal cells for steroid-refractory acute GvHD. Bone Marrow Transplant. 2017;52:1577–9.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Sánchez Casado M, Quintana Díaz M, Palacios D, Hortigüela V, Marco Schulke C, García J. et al. [Relationship between the alveolar-arterial oxygen gradient and PaO2/FiO2-introducing PEEP into the model]. Med intensiva. 2012;36:329–34.
Gattinoni L, Vassalli F, Romitti F. Benefits and risks of the P/F approach. Intensive Care Med. 2018;44:2245–7.
Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care. 2022;26:1–14.
Kirkham AM, Bailey AJM, Shorr R, Lalu MM, Fergusson DA, Allan DS. Systematic review and meta-analysis of randomized controlled trials of mesenchymal stromal cells to treat coronavirus disease 2019: is it too late? Cytotherapy. 2023;25:341–52.
Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–12.
Liang D, Liu C, Yang M. Mesenchymal stem cells and their derived exosomes for ALI/ARDS A promising therapy. Heliyon. 2023;9:e20387.
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–28.
Sánchez-Guijo F, García-Arranz M, López-Parra M, Monedero P, Mata-Martínez C, Santos A, et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454.
Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020;53:1–8.
Hashemian SMR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12:1–12.
Xu X, Jiang WW, Chen LLLL, Xu Z, Zhang Q, Zhu M, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin Transl Med. 2021;11:e297.
Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA. et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–73.
Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361.
Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6:58.
Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30:1–14.
Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl Med. 2021;10:1279–87.
Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, Stimamiglio MA, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13:1–22.
Zhu R, Yan T, Feng Y, Liu Y, Cao H, Peng G, et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021;31:1244–62.
Couto PS, Al-arawe N, Filgueiras IS, Fonseca DLM, Hinterseher I, Catar RA, et al. Systematic review and meta-analysis of cell therapy for COVID-19: global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery. Front Immunol. 2023;14:1200180.
Liu Q, Ma F, Zhong Y, Wang G, Hu L, Zhang Y, et al. Efficacy and safety of human umbilical cord—derived mesenchymal stem cells for COVID ‑ 19 pneumonia: a meta ‑ analysis of randomized controlled trials. Stem Cell Res Ther. 2023;14:118.
Shi L, Yuan X, Yao W, Wang S, Zhang C, Zhang B, et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:1–14.
Acknowledgements
We would like to acknowledge the generous contribution of volunteer donors of bone marrow for MSC production. In addition, we would also like to recognize the valuable and selfless dedication of all healthcare professionals who helped looking after COVID-19 patients, and contributed to generate scientific evidence, despite the difficulties imposed by the pandemic and its several waves.
Funding
This study was partly funded by the “Transferencia Nominativa COVID-19” of the Consejería de Sanidad de la Comunidad de Madrid, by the Fundación ACS and by the “Proyecto Mascarillas Boticaria”. These parties did not participate in the conduct of the study or the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MEMM, CPH, IL, RMM, IS, RA, CAS and RFD: Conception and design. MEMM, CPH, IS, and RA: Collection and/or assembly of data. MEMM, CPH, IS, RA, CAS and RFD: data analysis and interpretation. MEMM, CPH and RFD: manuscript writing. IL, RMM, ATA, MPT, AR, PU and JJR: Provision of study material or patients and clinical follow-up. TMD, RS, RZ, MGB: MSC manufacture. CAS: sponsor’s regulatory responsibilities. IL and RFD: funding acquisition. All authors critically reviewed the manuscript and accepted the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This trial adhered to the Declaration of Helsinki and received ethics approval with the title “Ensayo clínico doble ciego, aleatorizado, controlado, para evaluar la eficacia de las células mesenquimales estromales alogénicas en pacientes con síndrome de distrés respiratorio agudo debido a COVID-19 (COVID-AT)”, on June 3rd 2020, from the local research ethics committee “Comité de Ética de la Investigación con medicamentos del HUPHM”, with approval number 82-20. Informed consent was obtained prior to performing any study procedures from all patients or their representatives (witnessed oral consent, documented in writing).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez-Muñoz, M.E., Payares-Herrera, C., Lipperheide, I. et al. Mesenchymal stromal cell therapy for COVID-19 acute respiratory distress syndrome: a double-blind randomised controlled trial. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02230-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02230-5