Abstract
Late-life depression has been consistently associated with lower gray matter volume, the origin of which remains largely unexplained. Recent in-vivo PET findings in early-onset depression and Alzheimer’s Disease suggest that synaptic deficits contribute to the pathophysiology of these disorders and may therefore contribute to lower gray matter volume in late-life depression. Here, we investigate synaptic density in vivo for the first time in late-life depression using the synaptic vesicle glycoprotein 2A receptor radioligand 11C-UCB-J. We included 24 currently depressed adults with late-life depression (73.0 ± 6.2 years, 16 female, geriatric depression scale = 19.5 ± 6.8) and 36 age- and gender-matched healthy controls (70.4 ± 6.2 years, 21 female, geriatric depression scale = 2.7 ± 2.9) that underwent simultaneous 11C-UCB-J positron emission tomography (PET) and 3D T1- and T2-FLAIR weighted magnetic resonance (MR) imaging on a 3-tesla PET-MR scanner. We used analyses of variance to test for 11C-UCB-J binding and gray matter volumes differences in regions implicated in depression. The late-life depression group showed a trend in lower gray matter volumes in the hippocampus (p = 0.04), mesial temporal (p = 0.02) and prefrontal cortex (p = 0.02) compared to healthy control group without surviving correction for multiple comparison. However, no group differences in 11C-UCB-J binding were found in these regions nor were any associations between 11C-UCB-J and depressive symptoms. Our data suggests that, in contrast to Alzheimer’s Disease, lower gray matter volume in late-life depression is not associated with synaptic density changes. From a therapeutic standpoint, preserved synaptic density in late-life depression may be an encouraging finding.
Similar content being viewed by others
Introduction
Late-life depression (LLD) is a major depressive disorder (MDD) in later life and has a poorly understood aetiology. Multiple lines of evidence suggest that accelerated biological brain aging might constitute an important pathway to the pathogenesis of LLD. For example, LLD is associated with increased vascular pathology [1], enhanced molecular and senescent changes [2], increased neuroinflammation [3], and lower gray matter volume (GMV) [4]. With regard to the latter, magnetic resonance imaging (MR) studies of late-life depression consistently report lower GMV in brain regions also associated with Alzheimer’s Disease (AD) pathology, such as the frontal [5], temporal [6] and parietal regions [7]. The extent of GMV changes, however, are more pronounced in AD [8, 9]. Importantly, LLD is associated with a twofold risk of developing dementia [10]. Yet the chain of events leading to lower gray matter volume in late-life depression is still unresolved.
Recent research in MDD points towards synapse loss as a mechanism for neural loss [11]. Emerging antidepressant therapies, including ketamine [12], psilocybin [13] and electroconvulsive therapy [14] are thought to enhance synaptic plasticity and have shown promising clinical results in depression [15, 16]. Interestingly, synapse loss is an early feature of mesial temporal atrophy in AD [17, 18], possibly explaining the clinical overlap between LLD and AD [19]. Imaging of synaptic density might therefore shed light on potential neurodegenerative patterns in LLD.
Quantifying synaptic density in humans in vivo recently became possible with the advent of radioligands targeting the synaptic vesicle glycoprotein 2A (SV2A), a ubiquitous presynaptic transmembrane protein [20]. Hence, imaging of SV2A using 11C-UCB-J positron emission tomography (PET) provides a suitable proxy for synaptic density in both physiological and pathological conditions [21]. Holmes et al. reported an inverse relationship between depression severity and hippocampal, prefrontal and anterior cingulate 11C-UCB-J binding in a group of depressed adults under the age of 60 with and without post-traumatic stress disorder (PTSD) [22]. Whether these findings are generalizable to LLD and whether they might be linked to lower GMV merits elucidation. We therefore conducted a cross-sectional case-control study in older adults that investigated synaptic density and GMV in brain regions implicated in LLD [4]. We hypothesized that GMV and 11C-UCB-J binding would be lower in the hippocampus, mesial temporal cortex, anterior cingulate cortex, lateral temporal cortex, prefrontal cortex and parietal cortex in LLD compared to heathy controls, and that synaptic density would be inversely associated with depression severity.
Materials and Methods
Participants
All participants from the ongoing Leuven Late Life depression study (see [23] for detailed information regarding sample size and recruitment) enrolled before November 2022 were included. Based on the only 11C-UCB-J study investigating differences in depression (MDD not LLD) compared to healthy controls, achieving 80% power at an α-level of .008 requires 12 subjects per group. Our LLD group consists of patients over 60 years of age referred to the geriatric psychiatry unit at the University Psychiatric Center (UPC) KU Leuven hospital for unipolar depression as the primary diagnosis, and without any major comorbidity. All fulfilled DSM-5 criteria for major depressive disorder as clinically assessed by a psychiatry resident (TVC) at the time of inclusion. Our healthy control (HC) group consists of 36 age- and gender-matched older adults in the same age range. Exclusion criteria for both groups were screened during a formal medical interview and covered the history or presence of any major disease that may interfere with the investigations, history or presence of a major neurological disorder, history or presence of a psychiatric disease (except depressive episodes for the LLD group), past or current drug or alcohol abuse, and any present contra-indication for PET or MR scanning. The study was approved by the Ethics Committees of the University Hospitals (UZ) Leuven and UPC-KU Leuven (S61968). In accordance with the latest version of the Declaration of Helsinki, all participants provided written informed consent at study entry.
Clinical assessment and ApoE genotyping
All participants underwent a structured neuropsychiatric interview and neuropsychological testing. We used the Geriatric Depression Scale (GDS, 30 items) [24] and the Montgomery-Asberg Depression Rating Scale (MADRS) [25] to assess depression severity, the Mini-Mental State Examination (MMSE) [26] to assess global cognitive function and the Rey Auditory Verbal Learning Test [27] immediate recall trials (RAVLT-IR) as a proxy for episodic memory. All participants were genotyped to determine apolipoprotein ε (ApoE) status.
Imaging acquisition and preprocessing
PET and MR brain imaging data were simultaneously acquired on GE Signa 3 T time-of-flight (TOF) PET-MR (GE Healthcare, Milwaukee, WI, USA).
MR acquisition
High-resolution 3D BRAVO T1-weighted images (plane: oblique; TE: 3.2 ms; TR: 8.5 ms; TI: 450 ms; Flip Angle: 12; slice thickness: 1 mm, voxel size 1x1x1 mm3; and 3D T2-weighted FLAIR images (plane: oblique; TE: 137 ms; TR: 8500 ms; TI: 2300 ms; Flip Angle: 12; slice thickness: 1 mm, voxel size 1x1x1 mm) were acquired with a 32-channel head coil on the PET-MR. T1-weighted images were segmented by the default segmentation pipeline of the CAT12 toolbox (v12.6-rc1, Friedrich Schiller University Jena, Jena, Germany) to obtain tissue probability maps. Gray matter volumes-of-interest (VOIs) implicated in LLD (hippocampus, mesial temporal cortex, anterior cingulate cortex, lateral temporal cortex, prefrontal cortex and parietal cortex) were delineated in native space by masking the neuromorphometrics atlas (Neuromorphometrics, Inc) with gray matter probability map thresholded at 0.3. Since we did not expect laterality effects, left and right VOIs were merged into bilateral VOIs. All T1-weighted images were rated as “good (B)” by the quality control scale from CAT12, and no abnormalities were observed following visual assessment. Total intracranial volume (TIV, including brain, meninges and cerebrospinal fluid) was estimated by CAT12. White matter (periventricular and deep) lesions (WML) were delineated automatically with an AI based algorithm implemented in icoBrain [28] applied to T1- and T2 FLAIR-weighted images before manual revision by a neurologist.
PET acquisition
11C-UCB-J GMP tracer production was done as described previously [18]. On average 182.2 ± 54.4 MBq of 11C-UCB-J was injected intravenously 60 minutes before a 30 minute static PET acquisition in list mode. By use of an iterative ordered-subset expectation maximization algorithm (28 subsets, 4 iterations) 6 PET frames of 5 minutes were reconstructed incorporating time of flight data, as well as decay, scatter, and a validated zero echo time (ZTE) attenuation correction [29]. We applied isotropic Gaussian smoothing (4 mm full width at half maximum) to improve signal-to-noise ratio. Off-scanner fully automated all-in processing was done with inhouse scripts (https://github.com/THOMVDC/PSYPET) written in MATLAB (MathWorks, Natick, Mass.) and solely based on SPM12 (v7771, Wellcome Trust Centre for Neuroimaging, University College London) and CAT12 (v12.6-rc1, Friedrich Schiller University Jena, Jena, Germany) to obtain standardized uptake value ratio images (SUVR, Fig. 1). Processing of the 6 PET frames included the following steps: motion correction and averaging of the frames, standardized uptake value (SUV) calculation, and co-registration of the SUV image to the anatomical T1-weighted image. The neuromorphometrics atlas VOI delineation of the anatomical image was projected onto each corresponding SUV image. Since expected GMV differences might induce partial volume effects [30], we corrected images with an validated inhouse region-based voxel-wise partial volume correction (RBV PVC) algorithm (see Mertens et al. [31]. for a detailed description) using the neuromorphometrics VOIs as input regions. The centrum semiovale served as a reference region to calculate SUVR images (see Michiels et al. [32]. for the delineation method) maps after excluding any white matter lesions from the reference region (see MR acquisition for delineation method) [32, 33].
Statistical analysis
We performed all statistical testing with Python 3.6.8 built-in packages (python.org) as well as SciPy 1.4.1 (scipy.org) and Pingouin (pingouin-stats.org) 0.3.12 packages. Distributions of variables were verified for normality using histograms, q-q plots, and Shapiro-Wilk tests. Mild outliers, defined as beyond 1.5 times the interquartile range (IQR), were included in the imaging analysis. Clinical characteristics were analyzed with descriptive statistics using the chi-squared test (sex), Fisher exact test (apoE e4 carriers, amyloid positivity rate) independent Student t-test (11C-UCB-J binding in reference region), or Mann-Whitney U test (all others). To detect VOI-based GMV group differences, we applied analysis of covariance (ANCOVA) with age as a covariate after normalizing the volume of the VOIs by the total intracranial volume (1000‰ x volumeVOI / volumeTIV) [34]. Group differences in 11C-UCB-J SUVR values were evaluated with analysis of variance (ANOVA) without age as a covariate since age has no a priori influence on 11C-UCB-J binding [35] and there were no significant group differences in age. Partial eta squared was used as a measure of effect size in the group comparisons. Clinical correlations of depression severity, cognitive function and episodic memory with SUVR data in LLD were assessed by Pearson’s r, and clinical correlations with volumetric data in LLD were assessed by Pearson’s r controlling for age. Significant findings were claimed after Bonferroni correction for multiple comparisons (α = 0.008). Given the exploratory nature of our study, we also report findings for a threshold of α = 0.05 as a trend while clearly stating that these are not surviving Bonferroni correction. In addition, we performed post hoc analyses of 11C-UCB-J binding differences in (1) late-onset (first episode after 60 years) versus early-onset depression (<60 years) as tested with Mann-Whitney U tests, (2) depression with versus without psychotic features as evaluated with Mann-Whitney U tests, (3) depression with low or high severity of depressed symptoms versus HC based on GDS score (=>20 : severe depression (n = 13), <20: mild depression (n = 11)), (4) unilateral VOIs in LLD versus HC as tested with ANOVA, (5) LLD versus HC as tested with a voxel-based analysis inside the merged VOIs using SPM12 with a significance level pheight, FEW-corrected < 0.05 and a cluster extent kE ≥ 50 voxels, (6) LLD versus HC for non-partial volume corrected VOI data (7) LLD versus HC using the reference region without subtracting WML (7) LLD patients taking benzodiazepines vs benzodiazepine naïve LLD patientsLastly (8), a causal mediation analysis was carried with the mediation 4.5.0 package in R 4.0.2 to investigate whether the association between late-life depression and gray matter volume is mediated by synaptic density. Related to this analysis, we also assessed correlations between SUVR and GMV using Pearson’s r, corrected for age.
The experimental procedures (image acquisition and image processing) were performed once and the main statistical analyses were performed three times as a quality assurance check.
Results
Demographics and clinical data
We analyzed data from 24 LLD patients (age 73.0 ± 6.2 years, 16 female) and 36 age- and gender-matched healthy controls (age 70.4 ± 6.2 years, 21 female). An additional five healthy controls and ten patients enrolled in the L3D study were excluded from the analysis based on incomplete PET-MR scanning. Within a range of [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] on the GDS and a range of [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] on the MADRS, depression rating scale scores were significantly higher in the LLD group (p < 0.0001) (Table 1); 13 LLD patients scored equal or higher than the moderate-severe depression cutoff (20/30) on the GDS. Eight patients had MDD with psychotic features. At the time of inclusion, all but one LLD patient received antidepressant medication (supplementary table 1) and twelve patients took benzodiazepines. In comparison, no HC took antidepressants. Fifteen patients had at least one early depressive episode (before 60 years), one patient had a previous late-onset episode, and eight patients had never been depressed before the current episode. LLD patients scored significantly lower on the MMSE and RAVLT-IR (p < 0.0001). ApoE e4 status did not differ between both groups, the proportion of ApoE e4 carriers was within a normal reference range. PET-MR scanning followed inclusion in a median time of 7 days (IQR = 11) for the LLD group, 35 days (IQR = 119) for the healthy control group.
Volumetric group differences
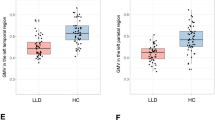
We found lower GMV in the LLD group compared to the HC group in the hippocampus (F(1,57) = 4.56, p = 0.04, ηp2 = 0.074), mesial temporal (F(1,57) = 6.11, p = 0.02, ηp2 = 0.097) and prefrontal VOIs (F(1,57) = 6.33, p = 0.02, ηp2 = 0.10), but not in the anterior cingulate (F(1,57) = 0.55, p = 0.46, ηp2 = 0.0095), lateral temporal (F(1,57) = 4.02, p = .05, ηp2 = .066) and parietal VOIs (F(1,57) = 2.30, p = 0.13, ηp2 = 0.039). None of the ANCOVA results survived Bonferroni correction (Fig. 2A, supplementary table 2A).
Volume-of-interest (VOI) boxplots of (A) by total intracranial volume (TIV) normalized grey matter volumes (‰) and (B) mean 11C-UCB-J SUVRs in the late-life depression (LLD) and healthy control (HC) groups. Boxes represent the interquartile range of the datapoints, whiskers represent the range of the datapoints except for outliers (>1.5 IQR). The range of the Y-axis of plot A is rescaled for the last three VOIs to fit the figure size. The LLD group showed lower normalized grey matter volumes in the hippocampus (*), mesial temporal VOI (*) and prefrontal VOI (*) compared to HC (without surviving Bonferroni correction). The LLD group did not show any differences of 11C-UCB-J binding in all VOIs.
Synaptic density group differences
We did not find any differences in 11C-UCB-J binding in the LLD group compared to the HC group in the hippocampus (F(1,58) =0.11, p = 0.74, ηp2 = 0.0019), mesial temporal (F(1,58) = 3.98, p = 0.05, ηp2 = 0.064), anterior cingulate (F(1,58) = 2.99, p = 0.09, ηp2 = 0.049), lateral temporal (F(1,58) = 1.54, p = 0.22, ηp2 = 0.026), prefrontal (F(1,58) = 1.85, p = 0.18, ηp2 = 0.031) and parietal VOIs (F(1,58) =0.33, p = 0.56, ηp2 = 0.0057) (Fig. 2B, Supplementary Table 2B).
Clinical correlations in LLD
Volumetry
Within the LLD group, age correlated with GMV in the hippocampus (r = −0.47, p = 0.02), mesial temporal (r = −0.42, p = 0.04) and parietal VOIs (r = −0.62, p = 0.001) but not in the anterior cingulate VOI, lateral temporal VOI and prefrontal VOI (Table 2). Only the association between age and parietal gray matter volume survived Bonferroni correction. Partial correlations correcting for age did reveal a correlation between the parietal GMV and the GDS (r = 0.45, p = 0.03), the MADRS (r = 0.44, p = 0.03) and the MMSE (r = 0.45, p = 0.03) without surviving Bonferroni correction. Prefrontal GMV showed a correlation with the RAVLT-IR (r = 0.56, p = 0.01) without surviving Bonferroni correction. No correlations were found between the RAVLT-IR and GMV in the hippocampus, mesial temporal, anterior cingulate, lateral temporal and parietal VOIs after correcting for age. The scatter plots associated with these results can be found in the supplementary materials (Supplementary Figs. 1, 2 and 3).
Synaptic density
Within the LLD group, age and depression severity did not correlate with 11C-UCB-J SUVR in any of the VOIs. A negative correlation was found between 11C-UCB-J SUVR and MMSE in the prefrontal (r = −0.48; p = 0.02) and the parietal VOI (r = −0.44, p = 0.03) without surviving Bonferroni correction, but not in hippocampus, mesial temporal and lateral temporal VOIs. (Table 3) No correlations were found between RAVLT-IR and 11C-UCB-J SUVR values in all VOIs.
Post hoc analyses
Post hoc analyses of 11C-UCB-J SUVR values in late-onset (after 60 years) versus early onset (before 60 years) depression, and between LLD without versus with psychotic features did not reveal any group differences (supplementary tables 3 and 4). No group differences in 11C-UCB-J SUVR were found between low-severity LLD and HC, or between high-severity LLD and HC (supplementary table 5A and 5B). No significant 11C-UCB-J clusters were found in the voxel-based analysis inside the merged VOIs. SUVR data not corrected for partial volume effect in LLD vs HC showed similar results to the partial volume corrected data (supplementary table 6). Using the centrum semiovale without subtracting WML as a reference region did neither affect the SUVR results (supplementary table 7 and supplementary figure 4). No significant 11C-UCB-J binding differences were found between LLD patients taking benzodiazepines or not (supplementary table 8). Unilateral VOI analysis showed higher 11C-UCB-J binding in the left mesial temporal lobe of LLD participants without surviving Bonferroni correction (supplementary table 9). The association between LLD and gray matter volume was not mediated by synaptic density (supplementary table 10A), nor were there any associations between GMV and SUVR in any VOI (supplementary table 10B).
Discussion
We found no evidence for altered synaptic density in late-life depression as measured by 11C-UCB-J in brain regions implicated in depression, while some of the regions showed lower GMV in the LLD group (without surviving Bonferroni correction). Parietal GMV correlated with depressive scales and the MMSE, without surviving Bonferroni correction. Prefrontal GMV correlated with the RAVLT-IR, without surviving Bonferroni correction. VOI-based correlations between 11C-UCB-J binding and depressive scales could not establish a link between synaptic density and depression severity, whilst there was some evidence for an inverse correlation with MMSE in the PFC and parietal cortex.
Until recently the hypothesis linking synaptic density with depression had only been investigated ex-vivo in animal stress studies and post-mortem depression studies focusing on the hippocampus and prefrontal cortex [11, 36]. Cell-counting studies incriminated neuronal and glial abnormalities for lower gray matter volume in MDD [37], while histopathological findings showed lower prefrontal synapse-related gene expression hinting at lower synaptic density in MDD [36]. Our finding suggests that there is no regional difference in synaptic density as measured by 11C-UCB-J across several cortical regions implicated in late-life depression. This does not preclude alterations in synaptic function since 11C-UCB-J might not capture vesicle release dynamics [38]. In contrast, preserved synaptic density might indicate that synaptopathy on a structural level is not a key pathophysiological feature in LLD, and possibly allows reversibility of network dysfunctions. This is in line with the extensive literature indicating a favorable outcome after adequate treatment in LLD [39].
The only other study that has investigated 11C-UCB-J in depression focused on unmedicated depressed MDD/PTSD adults under the age of 60 [22]. They reported lower distribution volumes (VT, representing specific and unspecific binding) of 11C-UCB-J in the depressed group within the hippocampus, anterior cingulate cortex and dorsolateral prefrontal cortex, as well as other regions not assessed in their primary analysis: occipital cortex, parietal cortex, temporal cortex, cerebellum, putamen. There are a number of reasons that make a direct comparison of results challenging. First, the study population differed in terms of age (39.2 ± 12.1 years versus 73.0 ± 6.2 years), diagnosis (MDD/PTSD mixed group versus LLD only), and medication use (unmedicated versus medicated). Aside from clinical heterogeneity, the respective PET quantification methods differed, but were both derived from preclinical validation studies.
GMV in the LLD group was lower in the hippocampus, mesial temporal and prefrontal VOI (none surviving Bonferroni correction). The prefrontal cortex, in concert with the hippocampus, also modulates memory processing in a top-down manner [40]. Episodic memory dysfunction is often reported in late-life depression and correlates well with depression severity [41]. Consistent with the brain aging literature, we found a correlation between age and hippocampal, mesial temporal and parietal gray matter volume (only parietal VOI survived Bonferroni correction).
Gray matter volume decrease is well described in aging [42], but it is not necessarily correlated with a loss of neurons [43] or synaptic density decrease (as measured by 11C-UCB-J) [35]. This is in contrast to most neurodegenerative diseases, such as AD where pathology leads to a net loss of synapses [44] and eventually to a loss of neurons and to gray matter volume decrease [17]. In AD the atrophy rate has been estimated to 1-4% a year [45] and has been linked to synaptic density (as measured by 11C-UCB-J) decreases even in early stages [46]. If lower GMV in LLD was associated with neurodegenerative-related changes, one would expect lower synaptic density to precede the volumetric changes. In contrast, our findings suggest that even when gray matter volume is lower, net synaptic loss does not exceed net volume loss such that synaptic density remains stable, in a comparable way to age-related gray matter volume loss [35].
Distinguishing etiological and consequential factors that contribute to the clinical presentation of LDD is challenging given the reciprocal relationships between most of these factors. Accordingly, deviation from normative GMV trajectories in LLD [47] might result from or cause LLD in a synergistic manner. The hypothesis of accelerated (brain) aging associated with LLD [48] might explain lower GMV without changes in synaptic density. Age-related GMV decrease might be essentially driven by a reduction in microglia and astrocytes [49]. In addition, cardiovascular and proinflammatory backgrounds that are common in LLD might contribute to GMV reduction without affecting synaptic density [50].
Another (non-exclusive) explanation might be that lower GMV is pre-existing to depression onset. Specifically, lower GMV at baseline does not imply lower density of synapses in that tissue. Lower GMV has been associated with less cognitive, executive and coping reserve towards life stressors [51] and might therefore constitute a vulnerability marker in an aging population.
This is the first prospective study measuring synaptic density in vivo in LLD. As it is challenging to motivate this population for participation in demanding imaging studies, research in the field might be biased towards milder depression. Mild depressive symptoms in later life might be a prodrome of neurodegenerative diseases [52], where synaptic density decrease is increasingly described [53]. Our cohort consisted of patients referred to the geriatric psychiatry unit with a major depressive disorder as the primary diagnosis, and without any major neurological comorbidity. In these terms, preserved 11C-UCB-J in late-life depression might be a potential differential diagnostic biomarker between depression as a primary psychiatric disorder and depressive symptoms as a consequence of neurodegenerative disorder.
Some methodological study limitations to the present study are important to highlight. T1-weighted MR segmentation is prone to artefacts and epiphenomena [54], yet the differences in MR measurements we reported in LLD are in line with histopathologic literature and therefore suggestive for GMV differences. We used a simplified quantification method (SUVR) instead of BPND or DVR as SUVR calculation does not require invasive arterial sampling nor dynamic scanning up to 90 min but only a 30 min scan, thus increasing (clinical) feasibility of the protocol in depression. This quantification method has been validated in healthy aging [33] and in AD [55], but not in LLD. Second, since LLD is often associated with vascular burden, we cannot exclude white matter lesions (WML) having biased the present results. To reduce impact from WML on 11C-UCB-J binding in the centrum semiovale (CS), we subtracted WML from the CS masks (see PET acquisition, supplementary table 11). Using the CS without correction for WML as a reference region did not affect the main outcome; no group differences in mean SUVR values were found in all VOIs (supplementary Table 7).
Another limitation arises in the interpretation of 11C-UCB-J as a proxy for synaptic density. The extent to which SV2A binding reflects the entire pool of presynaptic vesicles, and to what extent the labeling of vesicles is dependent on the current physiological state of the synapse requires further validation [21, 38, 56]. Caution is also required when assuming that the number of presynaptic proteins SV2A is directly proportional to the number of synapses. Lastly, little is known about how psychotropic medication interferes with the binding of 11C-UCB-J. Except for an in vivo study with ketamine [57] and an in vitro study with lithium [58], which both had no effect on SV2A density in humans, it is not known how other antidepressants affect 11C-UCB-J binding. One theoretical concern is the effect of allosteric modulators of GABA-A, such as benzodiazepines, on SV2A distribution in GABAergic neurons. Post hoc analysis showed no intragroup differences between LLD patients taking benzodiazepines or not (supplementary table 8). Finally, whilst our power calculation was based on the only 11C-UCB-J study in depression (MDD/PTSD not LLD), we cannot exclude type II errors due to sample size and heterogeneity, for example, due to differences in medication use, symptom severity or the presence of anxiety, which we did not assess with formal rating scales.
In conclusion, we reproduced the finding of a trend in lower gray matter volume in regions implicated in late-life depression but found no evidence for altered synaptic density in those regions. In contrast to AD, lower gray matter volume in LLD seems not associated with lower synaptic density. Preserved synaptic density may suggest that the aging brain in LLD retains its capacity for neuroplasticity and remains amenable to therapeutic interventions. Future studies should replicate these preliminary findings and could investigate the clinical value of synaptic density as a differential diagnostic biomarker for LLD not associated with neurodegenerative diseases.
Data availability
All data that support the findings of this study are stored in a secured online research platform. Anonymized data are available upon reasonable request and approval by the local Ethics Committee.
Code availability
PET image processing was conducted using the publicly available PSYPET 167 tool, version 1 which was developed by the author TVC. It is freely available to download from https://github.com/THOMVDC/PSYPET.
References
Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–5.
Diniz BS, Mulsant BH, Reynolds CF, Blumberger DM, Karp JF, Butters MA, et al. Association of Molecular Senescence Markers in Late-Life Depression With Clinical Characteristics and Treatment Outcome. JAMA Netw Open. 2022;5:e2219678.
Köhler CA, Freitas TH, Maes M, De Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scandinavica. 2017;135:373–87.
Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review and Meta-Analysis of Magnetic Resonance Imaging Studies in Late-Life Depression. Am J Geriatr Psychiatry. 2013;21:184–95.
Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39:397–406.
Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–64.
Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, et al. Gray matter changes in late life depression-a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–72.
Boccia M, Acierno M, Piccardi L. Neuroanatomy of Alzheimer’s Disease and Late-Life Depression: A Coordinate-Based Meta-Analysis of MRI Studies. J Alzheimer’s Dis. 2015;46:963–70.
Emsell L, Vanhaute H, Vansteelandt K, De Winter F-L, Christiaens D, Van Den Stock J, et al. An optimized MRI and PET based clinical protocol for improving the differential diagnosis of geriatric depression and Alzheimer’s disease. Psychiatry Res: Neuroimag. 2022;320:111443.
Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–2544.e2534.
Ousdal OT, Brancati GE, Kessler U, Erchinger V, Dale AM, Abbott C, et al. The Neurobiological Effects of Electroconvulsive Therapy Studied Through Magnetic Resonance: What Have We Learned, and Where Do We Go? Biol Psychiatry. 2022;91:540–9.
Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J Affect Disord. 2021;278:542–55.
Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28:844–51.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Vanhaute H, Ceccarini J, Michiels L, Koole M, Sunaert S, Lemmens R, et al. In vivo synaptic density loss is related to tau deposition in amnestic mild cognitive impairment. Neurology. 2020;95:e545–e553.
Takamiya A, Vande Casteele T, Koole M, De Winter F-L, Bouckaert F, Van Den Stock J, et al. Lower regional gray matter volume in the absence of higher cortical amyloid burden in late-life depression. Sci Rep. 2021;11:15981.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra396.
Serrano ME, Kim E, Petrinovic MM, Turkheimer F, Cash D. Imaging Synaptic Density: The Next Holy Grail of Neuroscience? Front Neurosci. 2022;16:796129.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529.
Emsell L, Laroy M, Van Cauwenberge M, Vande Casteele T, Vansteelandt K, Van Laere K, et al. The Leuven late life depression (L3D) study: PET-MRI biomarkers of pathological brain ageing in late-life depression: study protocol. BMC Psychiatry. 2021;21:64.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49.
Montgomery SA, Åsberg M. A New Depression Scale Designed to be Sensitive to Change. Br J Psychiatry. 1979;134:382–9.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Ryan JJ, Geisser ME. Validity and diagnostic accuracy of an alternate form of the Rey Auditory Verbal Learning Test. Arch Clin Neuropsychol. 1986;1:209–17.
Rakic M, Vercruyssen S, Van Eyndhoven S, de la Rosa E, Jain S, Van Huffel S, et al. icobrain ms 5.1: Combining unsupervised and supervised approaches for improving the detection of multiple sclerosis lesions. Neuroimage Clin. 2021;31:102707.
Schramm G, Koole M, Willekens SMA, Rezaei A, Van Weehaeghe D, Delso G et al. Regional Accuracy of ZTE-Based Attenuation Correction in Static [18F]FDG and Dynamic [18F]PE2I Brain PET/MR. Front Phys. 2019;7.
Lu Y, Toyonaga T, Naganawa M, Gallezot JD, Chen MK, Mecca AP, et al. Partial volume correction analysis for (11)C-UCB-J PET studies of Alzheimer’s disease. Neuroimage. 2021;238:118248.
Mertens N, Michiels L, Vanderlinden G, Vandenbulcke M, Lemmens R, Van Laere K, et al. Impact of meningeal uptake and partial volume correction techniques on 18F-MK-6240 binding in aMCI patients and healthy controls. J Cereb Blood Flow Metab. 2022;42:1236–46.
Michiels L, Mertens N, Thijs L, Radwan A, Sunaert S, Vandenbulcke M, et al. Changes in synaptic density in the subacute phase after ischemic stroke: A 11C-UCB-J PET/MR study. J Cereb Blood Flow Metab. 2022;42:303–14.
Koole M, van Aalst J, Devrome M, Mertens N, Serdons K, Lacroix B, et al. Quantifying SV2A density and drug occupancy in the human brain using [(11)C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur J Nucl Med Mol Imaging. 2019;46:396–406.
Whitwell JL, Crum Wr Fau -, Watt HC, Watt Hc Fau -, Fox NC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. Am J Neuroradiol. 2001;22:1483–9.
Michiels L, Delva A, Van Aalst J, Ceccarini J, Vandenberghe W, Vandenbulcke M, et al. Synaptic density in healthy human aging is not influenced by age or sex: a 11C-UCB-J PET study. NeuroImage. 2021;232:117877.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7.
Stockmeier CA, Rajkowska G. Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues Clin Neurosci. 2004;6:185–97.
Smart K, Liu H, Matuskey D, Chen M-K, Torres K, Nabulsi N, et al. Binding of the synaptic vesicle radiotracer [11C]UCB-J is unchanged during functional brain activation using a visual stimulation task. J Cereb Blood Flow Metab. 2021;41:1067–79.
Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9:188.
Eichenbaum H. Prefrontal–hippocampal interactions in episodic memory. Nat Rev Neurosci. 2017;18:547–58.
McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8.
Cox SR, Harris MA, Ritchie SJ, Buchanan CR, Valdés Hernández MC, Corley J, et al. Three major dimensions of human brain cortical ageing in relation to cognitive decline across the eighth decade of life. Mol Psychiatry. 2021;26:2651–62.
Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–50.
Jackson J, Jambrina E, Li J, Marston H, Menzies F, Phillips K, et al. Targeting the Synapse in Alzheimer’s Disease. Front Neurosci. 2019;13:735.
Sluimer JD, Vrenken H, Blankenstein MA, Fox NC, Scheltens P, Barkhof F, et al. Whole-brain atrophy rate in Alzheimer disease: Identifying fast progressors. Neurology. 2008;70:1836–41.
Vanderlinden G, Ceccarini J, Vande Casteele T, Michiels L, Lemmens R, Triau E, et al. Spatial decrease of synaptic density in amnestic mild cognitive impairment follows the tau build-up pattern. Mol Psychiatry. 2022;27:4244–51.
Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. 2022;604:525–33.
Lavretsky H Does Late-Life Depression Accelerate Aging? Am J Geriatr Psychiatry. 2022.
Vidal-Pineiro D, Parker N, Shin J, French L, Grydeland H, Jackowski AP et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep. 2020;10.
Kosyreva AM, Sentyabreva AV, Tsvetkov IS, Makarova OV. Alzheimer’s Disease and Inflammaging. Brain Sci. 2022;12:1237.
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7.
Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement. 2013;9:602–8.
Carson RE, Naganawa M, Toyonaga T, Koohsari S, Yang Y, Chen M-K, et al. Imaging of Synaptic Density in Neurodegenerative Disorders. J Nucl Med. 2022;63:60S–67S.
Weinberger R, Eugenia Radulescu D. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am J Psychiatry. 2016;173:27–33.
Tuncel H, Boellaard R, Coomans EM, Hollander-Meeuwsen MD, De Vries EFJ, Glaudemans AWJM et al. Validation and test–retest repeatability performance of parametric methods for [11C]UCB-J PET. EJNMMI Research 2022;12.
Rossi R, Arjmand S, Baerentzen SL, Gjedde A, Landau AM. Synaptic Vesicle Glycoprotein 2A: Features and Functions. Front Neurosci. 2022;16:864514.
Holmes SE, Finnema SJ, Naganawa M, Dellagioia N, Holden D, Fowles K, et al. Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol Psychiatry. 2022;27:2273–81.
Halff EF, Cotel MC, Natesan S, McQuade R, Ottley CJ, Srivastava DP, et al. Effects of chronic exposure to haloperidol, olanzapine or lithium on SV2A and NLGN synaptic puncta in the rat frontal cortex. Behav Brain Res. 2021;405:113203.
Acknowledgements
The authors would like to thank the UZ Leuven PET-MR technical team (in particular Kwinten Porters, Jef Van Loock), the radiopharmacy team for their skilled support, Kristof Vansteelandt for his statistical advice and all study participants for their contribution.
Funding
This study was supported by Research Foundation Flanders (FWO grant, FWO/G0C0319N), KU Leuven internal C2 funding (C24-18-095) and the Sequoia Fund for Research on Aging and Mental Health.
Author information
Authors and Affiliations
Contributions
All authors substantially contributed either to the conception of the work (MK, PD, SS, JVDS, FB, KVL, LE, MV), the acquisition (TVC, ML, MVC), the analysis of data (TVC) and/or the interpretation of data (TVC, MK, KVL, LE, MV). TVC drafted the work as a first author, all other authors revised the draft critically. All authors approve this version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
KVL performed this study as senior investigator of FWO (Fonds Wetenschappelijk Onderzoek) Flanders. KVL is an advisory board member of Cerveau and has received fees through KU Leuven for consultancy activities for GE Healthcare. KVL has performed contract research through KU Leuven. All other authors have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vande Casteele, T., Laroy, M., Van Cauwenberge, M. et al. Preliminary evidence for preserved synaptic density in late-life depression. Transl Psychiatry 14, 145 (2024). https://doi.org/10.1038/s41398-024-02837-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-02837-8