Abstract
Elevated brain glutamate has been implicated in non-response to antipsychotic medication in schizophrenia. Biomarkers that can accurately predict antipsychotic non-response from the first episode of psychosis (FEP) could allow stratification of patients; for example, patients predicted not to respond to standard antipsychotics could be fast-tracked to clozapine. Using proton magnetic resonance spectroscopy (1H-MRS), we examined the ability of glutamate and Glx (glutamate plus glutamine) in the anterior cingulate cortex (ACC) and caudate to predict response to antipsychotic treatment. A total of 89 minimally medicated patients with FEP not meeting symptomatic criteria for remission were recruited across two study sites. 1H-MRS and clinical data were acquired at baseline, 2 and 6 weeks. Response was defined as >20% reduction in Positive and Negative Syndrome Scale (PANSS) Total score from baseline to 6 weeks. In the ACC, baseline glutamate and Glx were higher in Non-Responders and significantly predicted response (P < 0.02; n = 42). Overall accuracy was greatest for ACC Glx (69%) and increased to 75% when symptom severity at baseline was included in the model. Glutamate metabolites in the caudate were not associated with response, and there was no significant change in glutamate metabolites over time in either region. These results add to the evidence linking elevations in ACC glutamate metabolites to a poor antipsychotic response. They indicate that glutamate may have utility in predicting response during early treatment of first episode psychosis. Improvements in accuracy may be made by combining glutamate measures with other response biomarkers.
Similar content being viewed by others
Introduction
Although antipsychotic medication can be highly effective in reducing the severity of symptoms of schizophrenia, there is significant variation in the degree to which symptoms will improve with treatment [1, 2]. In approximately one third of patients, symptoms will not respond adequately to sequential treatment with non-clozapine antipsychotics and this subgroup are defined as meeting criteria for treatment resistant schizophrenia (TRS), for which the associated recommendation is to initiate treatment with clozapine [3, 4]. In a high proportion of TRS cases, a poor antipsychotic response may be present from illness onset [5], however currently TRS can only be identified retrospectively through failed treatment trials [4]. Furthermore, TRS is typically not identified until several years after TRS criteria have been met, so the initiation of clozapine is delayed, and during this time patients undergo ineffective treatment and prolonged duration of active symptoms. These delays in initiating adequate treatment are associated with poorer clinical outcomes [3, 6, 7]. Biomarkers that can accurately predict antipsychotic non-response from early after presentation with first episode psychosis (FEP) could aid clinical decisions to initiate clozapine earlier, and thereby greatly improve health and economic outcomes [8]. In addition, elucidating the neurobiology of non-response may lead to therapeutic targets for this group.
Brain glutamate is one potential biomarker of antipsychotic non-response [9]. Cross-sectional studies measuring brain glutamate using proton magnetic resonance spectroscopy (1H-MRS) have shown that non-remission of symptoms in FEP [10], TRS and clozapine-resistant schizophrenia [11,12,13,14,15] are associated with greater concentrations of glutamate metabolites in the medial frontal cortex (MFC)/anterior cingulate cortex (ACC). Meta-analysis has identified increased MFC/ACC glutamate metabolites in the TRS illness subtype [16] and mega-analysis of individual data from multiple studies in schizophrenia has shown that higher ACC glutamate metabolites are associated with more severe total and positive symptoms and worse functioning [17]. A previous prospective study found that ACC glutamate levels in minimally medicated FEP predicted subsequent non-remission following 4 weeks of treatment with amisulpride with 69% overall accuracy, increasing to 75% when age and baseline symptom severity were included in the model [18]. Other prospective studies in FEP have found that non-response is associated with higher glutamate metabolite concentrations in the striatum [19] or thalamus [20, 21]. Glutamate elevation may therefore contribute to poor treatment outcomes in schizophrenia, and this may be present from FEP. Accordingly, 1H-MRS glutamate metabolites could be a biomarker to predict treatment outcomes, which could be applied in the first weeks after presentation to aid clinical decision-making. Previous prospective studies examined glutamate in participants who were antipsychotic naïve or minimally medicated (<2 weeks) at baseline as a predictor of response to a single antipsychotic compound [18,19,20, 22]. It is not known whether these findings will generalise to the more naturalistic conditions of being measured within the first weeks of standard antipsychotic treatment.
Treatment with antipsychotic medication has been associated with reductions in glutamate in longitudinal studies [23, 24], including in the MFC/ACC [18, 25] and right caudate [26,27,28]. This hypothesis is supported by mega-analysis showing that higher doses of antipsychotics are associated with lower MFC glutamate concentrations [17]. Some studies have observed correlations between decreases in glutamate metabolites and symptomatic improvement [21, 23, 26, 28]. However, the elevation in MFC/ACC glutamate in patients who have not responded to equivalent or higher doses of antipsychotic medication [10,11,12,13,14,15] may indicate that reductions in glutamate metabolites on antipsychotic treatment are more likely to occur in treatment responsive illness. Potentially, early glutamatergic changes during initial antipsychotic treatment might better predict later clinical outcome, as has been suggested for changes in brain activity [29,30,31], but this has not yet been investigated.
In this observational study we examined glutamate metabolites in the ACC and striatum in a FEP cohort undergoing antipsychotic treatment over 6 weeks, across two study sites. Our primary hypothesis was that elevated glutamate metabolites at baseline would be associated with a poorer response to antipsychotic treatment. We also hypothesised that glutamate metabolites would be reduced during antipsychotic treatment, and that early reductions in glutamate metabolites over 2 weeks would be associated with a better clinical outcome at 6 weeks.
Methods
Participants
Data were collected as part of the ‘STRATA-2’ study, examining the biological effects of antipsychotic treatment, which had National Health Service Research Ethics Approval (Reference 17/NI/0209). Participants were recruited and assessed across two U.K. sites, King’s College London (KCL) and the University of Manchester (UoM). Patients gave written informed consent to participate. The study was open to patients 18–65 years of age, who were able to read and write in English at a level suitable to complete study procedures. Inclusion also required a DSM-5 diagnosis for schizophrenia, schizoaffective disorder, schizophreniform disorder or psychosis non-specified; being within the first 2 years of onset of the psychotic illness; minimal previous treatment with antipsychotic medication, defined as having received antipsychotic treatment for no longer than 4 weeks, after a period of being either antipsychotic naïve or antipsychotic-free for at least 14 days; and that at screening patients did not meet symptomatic criteria for remission [32]. Exclusion criteria included pregnancy or breastfeeding; meeting ICD-10 criteria for harmful substance misuse, psychotic disorder secondary to substance misuse or an organic brain disorder; treatment with clozapine in the last 3 months; history of severe head injury involving loss of consciousness for >5 min; the presence of standard contraindications to MRI at 3 Tesla or severe claustrophobia prohibiting MRI participation.
Study overview
The study included an initial screening visit followed by 3 assessment visits. The assessment visits were conducted within 4 weeks of commencing antipsychotic treatment (‘baseline’) and 2 and 6 weeks (each ± 7days) after baseline. Each assessment visit included MRI acquisition and clinical interview. Throughout the study participants continued to take antipsychotic medication as according to their normal clinical care.
Clinical and demographic interview
During the screening visit, following provision of informed consent, demographic information and medical history were recorded and the Mini International Neuropsychiatric Interview (MINI) was used to confirm diagnosis. At the screening and each assessment visit, symptom severity was evaluated using the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) [33], illness severity was evaluated using the Clinical Global Impression - Severity scale for schizophrenia (CGI-S), details of all prescription and non-prescription medications were recorded and adherence with antipsychotic medication was evaluated using the Clinician Rating Scale (CRS) [34].
1H-Magnetic Resonance Spectroscopy
Data were acquired at 3 Tesla on either a General Electric (GE) MR750 (General Electric Healthcare, Chicago, USA; KCL site) or a Philips Achieva (Philips Healthcare, The Netherlands; UoM site) magnetic resonance scanner. Following localiser and calibration sequences, sagittal T1-weighted (T1-w) accelerated ADNI-GO sequences were acquired as according to the details on the ADNI website http://adni.loni.usc.edu/methods/documents/mri-protocols/. T1-w images were automatically reformatted to provide axial, sagittal and coronal views. Voxel positioning was planned on the axial T1-w image and checked across all orientations. The centre of the 20 × 20 × 20 mm ACC voxel was positioned on the midline sagittal localizer 16 mm above the most anterior portion of the genus of the corpus callosum, with the most posterior edge of the voxel approximately 2 mm from the edge of the corpus callosum. The 20 × 20 × 20 mm right caudate voxel was positioned to include the maximal amount of caudate grey matter, and with the lower edge 3 mm dorsal to the anterior commissure [12]. Voxel positioning and an example spectrum from each site is shown in Supplementary Figs. 1 and 2. 1H-MRS data were acquired using Point RESolved Spectroscopy (PRESS), at a TE = 35 ms, TR = 2000 ms, 128 averages, bandwidth/sample frequency of ± 5000 Hz, 4096 complex points. On the GE system data were acquired using the standard GE PROton Brain Examination (PROBE) sequence. Unsuppressed water spectra were acquired in the same voxel locations. 1H-MRS details are provided in the minimum reporting standards for in vivo magnetic resonance spectroscopy checklist (Supplementary Table 1), according to consensus recommendations [35].
1H-MRS data processing and quality control
All imaging data were transferred to KCL for analysis. 1H-MRS spectra were analysed using LCModel version 6.3–1M [36] using the standard LCModel basis set, acquired using PRESS at 3 Tesla and 35 ms, and containing 16 metabolites (L-alanine, aspartate, creatine, phosphocreatine, GABA, glucose, glutamine, glutamate, glycerophosphocholine, glycine, myo-inositol, L-lactate, N-acetylaspartate, N- acetylaspartylglutamate, phosphocholine, taurine). All metabolite estimates were water referenced. Gannet version 3.0 (http://gabamrs.com) was used to co-register the voxels with the corresponding T1-w image to determine the voxel tissue fractions. Metabolite estimates (M) were corrected for the voxel fractions of white matter (WM), grey matter (GM) and cerebrospinal fluid (CSF) using the equation Mcorr = M(WM + 1.21GM + 1.55CSF)/(WM + GM) [37, 38]. The primary metabolite of interest was glutamate, reported here both as Glucorr, and as the summed signal of glutamate and glutamine (Glxcorr) in the ACC and in the caudate. Quality control for spectra followed our previously described procedure [12]. Individual metabolite estimates associated with Cramer Rao Lower Bounds (CRLB) > 20% were excluded.
Statistical analysis
To account for site effects, baseline 1H-MRS data were converted to z-scores prior to analyses, by subtracting the site mean from individual values before dividing by the site standard deviation using the data acquired in this study. Potential effects of demographic or clinical variables were investigated using t-tests or Pearson’s correlation analyses. Percentage reduction in PANSS total score was calculated after subtraction of minimum possible scores [39]. Responders were defined as patients who showed a > 20% reduction in PANSS total score from baseline to 6 weeks, and non-responders as a reduction of 20% or less. This aligns with the consensus recommendations of <20% symptom reduction over ≥6 weeks for identifying antipsychotic non-response during prospective treatment periods [4]. Binary logistic regression determined whether Glucorr and Glxcorr at baseline predicted response status at 6 weeks. The accuracy of significant prediction was evaluated by estimating the area under the receiver-operating curve (ROC). As percentage symptom reduction early into antipsychotic treatment can predict longer-term antipsychotic response [40,41,42] the percentage reduction in PANSS total score from baseline to 2 weeks was subsequently added to the model. Linear regression determined continuous relationships between baseline Glucorr and Glxcorr and the change in PANSS total score over 6 weeks, covarying for PANSS total score at baseline. Where results for PANSS total score were significant, the relationships with subscale scores were subsequently evaluated to determine the contributing symptom domains. Effects of time and 6-week response status on Glucorr and Glxcorr over the 3 assessment visits was examined using linear mixed effects modelling, accounting for random effects of site. For all analyses, the threshold for statistical significance was corrected to α = 0.025 to account for the two voxels of interest. Significance was not adjusted for the two glutamate measures (Glucorr and Glxcorr) as these are non-independent. Analyses were performed in SPSS version 27.
Results
A total of 89 patients were recruited to the study (Supplementary Fig. 3). Of these, 45 completed at least one MRI scan and the clinical assessments through to the 6-week follow-up (Table 1). Ten of these 45 participants had received previous antipsychotic medication; the reasons for cessation included adverse effects (n = 5), non-adherence (n = 4), or were unknown (n = 1). At 6 weeks, 27 patients were classified as responders and 18 were classified as non-responders. These groups did not differ in any clinical or demographic characteristics at baseline, or in antipsychotic chlorpromazine equivalents (CPZE) or adherence score on the CRS at 6 weeks (Table 1). Percentage reduction in PANSS total score from baseline to 2 weeks was associated with response status at 6 weeks (B = −0.03; S.E. = 0.01; Wald = 3.77; df = 1; P = 0.05; Exp(B) = 0.98).
1H-MRS data quality
MRI data were acquired in 53 patients at baseline, 38 patients at 2 weeks, and 39 patients at 6 weeks. 33 patients completed MRI at all 3 timepoints. ACC spectra were available in all scans acquired at each time-point. Caudate spectra were available in 45 of the 53 baseline scans, 36 of the 38 2-week scans, and 36 of the 39 6-week scans. Metabolite concentrations and spectral quality measures at each time-point are provided in Supplementary Tables 2 and 3. Baseline Glucorr and Glxcorr concentrations were normally distributed and did not differ by sex, age, or smoking (non-smoker vs daily smoker), alcohol, cannabis, other substance use, duration of psychosis nor antipsychotic CPZE at baseline (all P > 0.05).
Baseline glutamate metabolites and prediction of response
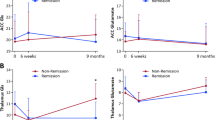
For the primary hypotheses testing the association between baseline glutamate metabolites and response at 6 weeks, data from 42 individuals were available for the ACC and 37 were available for the caudate. Both ACC Glucorr and Glxcorr at baseline significantly predicted response status at 6 weeks (Glucorr B = 0.88; S.E. = 0.38; Wald = 5.47; df = 1; P = 0.02; Exp(B) = 2.42; Glxcorr: B = 1.07; S.E. = 0.40; Wald = 7.33; df = 1; P < 0.01; Exp(B) = 2.92). This was related to increased concentrations of ACC Glucorr and Glxcorr in the Non-Responder compared to Responder group, with effect sizes of d = 0.82 and 1.02 respectively (Glucorr t40 = −2.62; P = 0.012; Glxcorr t40 = −3.27; P = 0.002, Table 2, Fig. 1). ROC analysis returned an area under curve of 0.72 for Glucorr and 0.75 for Glxcorr. Glucorr was associated with an overall accuracy of 62%, sensitivity of 66% (proportion of Responders correctly predicted to respond on the basis of baseline Glucorr), specificity 54% (proportion of Non-Responders correctly predicted not to respond on the basis of baseline Glucorr), positive predictive value (PPV) of 76% and a negative predictive value (NPV) of 41%. Glxcorr was associated with an overall accuracy of 69%, sensitivity 71%, specificity 64%, PPV 80% and NPV 53%. Including both ACC Glxcorr and early percentage change in PANSS Total score in the model increased the overall accuracy to 75%, sensitivity to 79%, specificity to 67%, PPV to 82% and NPV to 62%. The associations between baseline ACC Glucorr and Glxcorr and response status remained significant when covarying for antipsychotic CPZE dose or CRS scores at 6 weeks (P = 0.01 to 0.04). Caudate Glucorr and Glxcorr at baseline was not significantly associated with response status (Glucorr B = −0.13; S.E. = 0.32; Wald = 1.61; df = 1; P = 0.68; Exp(B) = 0.88; Glxcorr: B = 0.06; S.E. = 0.33; Wald = 0.03; df = 1; P = 0.87; Exp(B) = 1.06, Table 2, Fig. 1). None of the other metabolites showed significant associations with response (threshold P < 0.025, Table 2).
Baseline glutamate metabolites and continuous measures of response
ACC Glucorr and Glxcorr at baseline showed positive associations with the change in symptom severity over 6 weeks, such that lower levels of glutamate metabolites at baseline were associated with greater improvements in symptom severity over time (Fig. 2). There was a significant relationship between baseline ACC Glucorr and Glxcorr and change in PANSS Total score (Glucorr Beta = 0.50; t = 3.66; P < 0.001; Glxcorr Beta = 0.43; t = 4.39; P < 0.001) and this was also apparent across the majority of the PANSS symptom subscales (ACC Glucorr: PANSS Positive: Beta = 0.44; t = 3.51; P = 0.001; PANSS Negative: Beta = 0.18; t = 1.82; P = 0.08; PANSS General: Beta = 0.41; t = 3.39; P = 0.02; ACC Glxcorr PANSS Positive: Beta = 0.58; t = 3.97; P < 0.001; PANSS Negative: Beta = 0.25; t = 2.57; P = 0.01; PANSS General: Beta = 0.43; t = 3.72; P < 0.001). Covarying for CPZE dose or CRS at 6 weeks did not meaningfully alter these relationships. In the caudate, Glucorr and Glxcorr at baseline were not significantly associated with the change PANSS total scores over 6 weeks (P = 0.21 and 0.36). ACC and caudate Glucorr and Glxcorr at baseline were not significantly associated with PANSS Total score at baseline (P = 0.09 to 0.57).
Positive values for the change in PANSS represent increases in symptom severity. Glucorr glutamate, Glxcorr glutamate + glutamine, PANSS positive and negative syndrome scale for schizophrenia. The relationships between both ACC Glucorr and Glxcorr at baseline and change in PANSS Total score over 6 weeks were significant, P < 0.001.
Changes in glutamate metabolites over 6 weeks
There were no significant changes in glutamate metabolite concentrations over time, and the effect of time on glutamate metabolites also did not differ according to response status (measured at 6-weeks) (Supplementary Table 4; Fig. 3). There were no significant relationships between the symmetrized percentage change in glutamate metabolites over 2 or 6 weeks and response status or change in PANSS Total score at 6 weeks (all P > 0.025).
Discussion
In this observational, longitudinal study we examined glutamate metabolites in the ACC and striatum in a cohort of patients with FEP undergoing antipsychotic treatment according to their normal clinical care. The main finding was that higher levels of glutamate metabolites in the ACC were predictive of non-response to antipsychotic treatment, supporting our primary hypothesis. ACC glutamate metabolite levels at baseline were predictive of improvements across positive, negative, and general symptom domains. In contrast, glutamate metabolites in the caudate were not associated with response. We did not detect significant reductions in glutamate metabolites over time, nor associations between early glutamatergic change over 2 weeks and symptom severity at 6 weeks. Our results support the view that ACC glutamate elevations are associated with a poor antipsychotic response. With gains in predictive accuracy, ACC glutamate measures could form one component of a multivariate model to predict antipsychotic response from the first episode of psychosis.
The finding that elevated ACC glutamate metabolites are linked to a subsequently poor antipsychotic response is consistent with our previous prospective study examining FEP [18]. It is also accordant with the findings from most cross-sectional studies [10,11,12,13,14,15,16, 43] and meta-analysis [16] showing elevated ACC glutamate metabolites in antipsychotic nonresponsive patient groups. In contrast, two longitudinal studies in FEP have not found an association between elevated ACC glutamate metabolites at baseline and subsequent non-remission [20, 44] and one found the opposite relationship [22]. These studies examined antipsychotic naïve participants and one possibility is that antipsychotic exposure prior to baseline in our current and previous [18] cohorts may have elicited changes in glutamate, and this effect could potentially relate to associations with subsequent response. The latter study [22], examined a small voxel in the pgACC in an antipsychotic naïve cohort who had no history of substance use and reported lower glutamate levels in non-responders to risperidone, although this was not significant when glutamate was scaled to the internal reference of creatine-containing metabolites. One difference between our current and previous [18] studies and the two studies that did not find an association between ACC glutamate metabolites and response in FEP [20, 44] is that we examined a more rostral region of the ACC, corresponding to the perigenual ACC (pgACC). The pgACC is involved in internal mental processes and processing affective information [45, 46]. Glutamate concentrations are higher in rostral than caudal ACC regions [47] and differentially relate with whole brain resting state functional connectivity [48]. Early changes in pgACC connectivity predict antipsychotic response in FEP [30] and pgACC glutamate correlates with ACC control over sensory regions in antipsychotic-responsive but not treatment resistant schizophrenia [49]. Therefore, one possible explanation is that the mechanisms underlying antipsychotic response are localised to within the rostral, pgACC region.
Compared to some earlier research, our current study was designed to better reflect the possible ‘real-world’ conditions under which neuroimaging and other predictive biomarkers of response might be applied soon after first presentation. Prospective studies examining glutamate metabolites in relation to response in FEP have included participants who were antipsychotic naïve or had received minimal (< 2 weeks) antipsychotic exposure [18,19,20, 22, 44] and most examined a single antipsychotic compound [18,19,20, 22], whereas in our current study all participants were medicated at the baseline scan and antipsychotics were prescribed as usual by the treating clinical team.
Unlike in the ACC, we did not observe an association between caudate glutamate metabolites and subsequent antipsychotic response. Caudate glutamate metabolites also do not appear to differ in relation to response in cross sectional studies [12, 13, 15, 16]. One study reported higher striatal glutamate longitudinally in non-responders to risperidone, although this group difference was more apparent after risperidone treatment [19]. In meta-analysis, increases in glutamate metabolites in the striatum in schizophrenia are the most consistent finding across illness stages [16, 50], which indicates that they may be less sensitive to clinical variables and thus could provide a trait marker for schizophrenia spectrum disorders [16].
In contrast to some previous studies, including those in antipsychotic naïve/minimally medicated psychosis [18, 26, 27], we did not observe decreases in glutamate metabolites during antipsychotic treatment. Although systematic review and meta-analysis has also indicated ACC or striatal glutamate metabolite decreases related to antipsychotic treatment [16, 17, 23, 24] this is below significance in several individual studies [19,20,21, 51,52,53,54]. The relatively short observation period and inclusion of previously medicated patients may also contribute to why we did not observe these effects. The effects of antipsychotics on glutamate are likely to be indirect and mediated through mechanisms downstream of antipsychotic binding at D2/3 and other receptor subtypes. These indirect mechanisms may lead to more subtle changes, and more variability in effect related to differences in antipsychotic pharmacology [55], or other factors such as duration of administration or patient characteristics. Glutamatergic excess associated with antipsychotic non-response is more likely to be effectively reduced by novel therapeutics which directly target the glutamate system.
While our study was designed to provide greater generalisability to normal clinical care of FEP than prior studies, the presence of antipsychotic medication at baseline and lack of standardization of antipsychotic treatment may have added variability and reduced statistical power. The presence of antipsychotic medication at baseline limits comparison to previous studies that have enrolled antipsychotic-naïve participants [19, 20, 22] and prohibits inference about relationships between glutamate and clinical outcome in the absence of antipsychotic effects. The study design, requiring capacity to consent and willingness to participate in multiple MRI scans and clinical assessments, may have excluded more severely unwell patients and this group could also be less likely to respond to antipsychotic treatment. Data acquisition and analysis was harmonized across two sites, but the application of MRI platforms from different manufacturers was associated with site effects and may have reduced statistical power. Further discussion of 1H-MRS site effects and standardisation is available in Egerton et al. [12]. Since the start of this study, consensus recommendations have advocated the use of semi-adiabatic localisation by adiabatic selective refocusing (semi-LASER) over PRESS for 1H-MRS at 3 Tesla [56]. The 1H-MRS signal reflects the total amount of MR-visible glutamate in the voxel rather than glutamate neurotransmission specifically. Future studies examining changes in glutamate occurring in response to stimuli with functional 1H-MRS [57, 58] or combining 1H-MRS glutamate measures with functional connectivity [49], may provide greater mechanistic information about the role of glutamate in antipsychotic response and possibly greater predictive accuracy.
Our findings have potential clinical applications. Previous research has shown that when patients fail to respond to a first antipsychotic drug they are unlikely to respond to a second non-clozapine antipsychotic [59], and that the trial of a second antipsychotic rarely results in response, and merely introduces a delay in the use of clozapine, which in turn is associated with worse outcomes [6]. Accurate prediction of non-response during initial antipsychotic treatment could therefore identify a stratum of patients most likely to benefit from clozapine as a second line treatment. Hypothetical cost-effectiveness analysis indicates that predictive models with even modest sensitivity and specificity (over 60%) to indicate clozapine as a second line antipsychotic could achieve improvements in quality of life [8]. Baseline ACC glutamate or Glx was associated with values within this range, achieving up to 79% specificity and 67% sensitivity when the early percentage change in PANSS was included in the model. However, similarly to our previous study [18], antipsychotic responders were identified more accurately (maximum PPV: 82%) than non-responders (maximum NPV: 62%). This could be related to greater clinical or biological heterogeneity within the non-responder group, for example in some cases response may have emerged over a longer observation period. For the potential application of supporting earlier clozapine initiation in non-responders, improving the NPV is of clinical importance to avoid unnecessary clozapine initiation and the associated risks. Application would also require further optimization and standardization of 1H-MRS data acquisition, analysis and test-retest reliability across scanner manufacturers. Gains in accuracy could also potentially be achieved by combining glutamate measures with other predictors of response, such as resting state activity [60], brain volumes [9], perfusion [61], striatal dopamine synthesis capacity [62], cognitive performance [63] and clinical or demographic predictors [41, 42, 64].
In summary, this prospective study in FEP found that higher levels of glutamate metabolites in the ACC were predictive of non-response to antipsychotic treatment 6 weeks later. This finding supports the proposition that elevated ACC metabolites may be associated with a poor antipsychotic response. Combining 1H-MRS glutamate measures with other variables associated with antipsychotic response may increase predictive accuracy.
Data availability
At the time of submission, the data governance frameworks are being put in place to make a fully anonymized version of the data available to the wider research community. To apply for access to the data, please contact J.H.M. at james.maccabe@kcl.ac.uk
References
Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 2008;371:1085–97.
Boter H, Peuskens J, Libiger J, Fleischhacker WW, Davidson M, Galderisi S, et al. Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST). Schizophr Res. 2009;115:97–103.
Meltzer HY. Treatment-resistant schizophrenia–the role of clozapine. CurrMedResOpin. 1997;14:1–20.
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–29.
Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46:3231–40.
Griffiths K, Millgate E, Egerton A, MacCabe JH. Demographic and clinical variables associated with response to clozapine in schizophrenia: a systematic review and meta-analysis. Psychol Med. 2021;51:376–86.
Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62:975–83.
Jin H, McCrone P, MacCabe JH. Stratified medicine in schizophrenia: how accurate would a test of drug response need to be to achieve cost-effective improvements in quality of life? Eur J Health Econ. 2019;20:1425–35.
Gillespie AL, Samanaite R, Mill J, Egerton A, MacCabe JH. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry. 2017;17:12.
Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology 2012;37:2515–21.
Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in Schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2013;75:e11-13.
Egerton A, Murphy A, Donocik J, Anton A, Barker GJ, Collier T, et al. Dopamine and Glutamate in Antipsychotic-Responsive Compared With Antipsychotic-Nonresponsive Psychosis: A Multicenter Positron Emission Tomography and Magnetic Resonance Spectroscopy Study (STRATA). Schizophr Bull. 2021;47:505–16.
Iwata Y, Nakajima S, Plitman E, Caravaggio F, Kim J, Shah P, et al. Glutamatergic Neurometabolite Levels in Patients With Ultra-Treatment-Resistant Schizophrenia: A Cross-Sectional 3T Proton Magnetic Resonance Spectroscopy Study. Biol Psychiatry. 2019;85:596–605.
Mouchlianitis E, Bloomfield MA, Law V, Beck K, Selvaraj S, Rasquinha N, et al. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr Bull. 2016;42:744–52.
Tarumi R, Tsugawa S, Noda Y, Plitman E, Honda S, Matsushita K, et al. Levels of glutamatergic neurometabolites in patients with severe treatment-resistant schizophrenia: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2019;45:632–40.
Nakahara T, Tsugawa S, Noda Y, Ueno F, Honda S, Kinjo M, et al. Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of (1)H-magnetic resonance spectroscopy studies. Mol Psychiatry. 2022; 27:744–57.
Merritt K, McGuire PK, Egerton A, Investigators HMiS, Aleman A, Block W, et al. Association of age, antipsychotic medication, and symptom severity in Schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatry. 2021;78:667–81.
Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre (1)H-MRS study (OPTiMiSE). Mol Psychiatry. 2018;23:2145–55.
Reyes-Madrigal F, Guma E, Leon-Ortiz P, Gomez-Cruz G, Mora-Duran R, Graff-Guerrero A, et al. Striatal glutamate, subcortical structure and clinical response to first-line treatment in first-episode psychosis patients. Prog Neuropsychopharmacol Biol Psychiatry. 2022;113:110473.
Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naive patients with psychosis. Psychol Med. 2020;50:2182–93.
Merritt K, Perez-Iglesias R, Sendt KV, Goozee R, Jauhar S, Pepper F, et al. Remission from antipsychotic treatment in first episode psychosis related to longitudinal changes in brain glutamate. NPJ schizophrenia. 2019;5:12.
Li J, Ren H, He Y, Li Z, Ma X, Yuan L, et al. Anterior cingulate cortex glutamate levels are related to response to initial antipsychotic treatment in drug-naive first-episode schizophrenia patients. Front psychiatry. 2020;11:553269.
Egerton A, Bhachu A, Merritt K, McQueen G, Szulc A, McGuire P. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal 1H-MRS sStudies. Front psychiatry. 2017;8:66.
Kubota M, Moriguchi S, Takahata K, Nakajima S, Horita N. Treatment effects on neurometabolite levels in schizophrenia: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophr Res. 2020;222:122–32.
Goto N, Yoshimura R, Kakeda S, Nishimura J, Moriya J, Hayashi K, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–22.
de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA psychiatry. 2013;70:1057–66.
de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, et al. Prefrontal and striatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol Psychiatry. 2018;83:475–83.
McQueen G, Sendt KV, Gillespie A, Avila A, Lally J, Vallianatou K, et al. Changes in brain glutamate on switching to Clozapine in treatment-resistant schizophrenia. Schizophr Bull. 2021;47:662–71.
Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology 2009;34:2675–90.
Li H, Ou Y, Liu F, Chen J, Zhao J, Guo W, et al. Reduced connectivity in anterior cingulate cortex as an early predictor for treatment response in drug-naive, first-episode schizophrenia: A global-brain functional connectivity analysis. Schizophr Res. 2020;215:337–43.
Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 2014;39:1020–30.
Andreasen NC, Carpenter WT Jr., Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–49.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Kemp R, Hayward P, Applewhaite G, Everitt B, David A. Compliance therapy in psychotic patients: randomised controlled trial. BMJ 1996;312:345–9.
Lin A, Andronesi O, Bogner W, Choi IY, Coello E, Cudalbu C, et al. Minimum Reporting Standards for in vivo Magnetic Resonance Spectroscopy (MRSinMRS): Experts’ consensus recommendations. NMR Biomed. 2021;34:e4484.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn ResonMed. 1993;30:672–79.
Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–37.
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–26.
Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S. Defining ‘response’ in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology 2007;32:1903–10.
Kinon BJ, Chen L, Ascher-Svanum H, Stauffer VL, Kollack-Walker S, Sniadecki JL, et al. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res. 2008;102:230–40.
Stauffer VL, Case M, Kinon BJ, Conley R, Ascher-Svanum H, Kollack-Walker S, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187:42–8.
Derks EM, Fleischhacker WW, Boter H, Peuskens J, Kahn RS, Group ES. Antipsychotic drug treatment in first-episode psychosis: should patients be switched to a different antipsychotic drug after 2, 4, or 6 weeks of nonresponse? J Clin Psychopharmacol. 2010;30:176–80.
Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol. 2015;18:pyu117.
Dempster K, Jeon P, MacKinley M, Williamson P, Theberge J, Palaniyappan L. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol Psychiatry. 2020;25:1640–50.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44.
Dou W, Palomero-Gallagher N, van Tol MJ, Kaufmann J, Zhong K, Bernstein HG, et al. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J Neurosci. 2013;33:12698–704.
Li M, Danyeli LV, Colic L, Wagner G, Smesny S, Chand T, et al. The differential association between local neurotransmitter levels and whole-brain resting-state functional connectivity in two distinct cingulate cortex subregions. Hum Brain Mapp. 2022;43:2833–44.
Horne CM, Vanes LD, Verneuil T, Mouchlianitis E, Szentgyorgyi T, Averbeck B, et al. Cognitive control network connectivity differentially disrupted in treatment resistant schizophrenia. NeuroImage Clin. 2021;30:102631.
Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–74.
Kraguljac NV, Morgan CJ, Reid MA, White DM, Jindal RD, Sivaraman S, et al. A longitudinal magnetic resonance spectroscopy study investigating effects of risperidone in the anterior cingulate cortex and hippocampus in schizophrenia. Schizophr Res. 2019;210:239–44.
Birur B, Kraguljac NV, VerHoef L, Morgan CJ, Jindal RD, Reid MA, et al. Neurometabolic correlates of 6 and 16 weeks of treatment with risperidone in medication-naive first-episode psychosis patients. Transl psychiatry. 2020;10:15.
Jeon P, Limongi R, Ford SD, Mackinley M, Dempster K, Theberge J, et al. Progressive changes in glutamate concentration in early stages of schizophrenia: a longitudinal 7-Tesla MRS study. Schizophr Bull Open. 2021;2:sgaa072.
Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. BrJPsychiatry. 2007;191:325–34.
McLoughlin GA, Ma D, Tsang TM, Jones DN, Cilia J, Hill MD, et al. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. JProteomeRes. 2009;8:1943–52.
Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, et al. Methodological consensus on clinical proton MRS of the brain: Review and recommendations. Magn Reson Med. 2019;82:527–50.
Jelen LA, King S, Mullins PG, Stone JM. Beyond static measures: A review of functional magnetic resonance spectroscopy and its potential to investigate dynamic glutamatergic abnormalities in schizophrenia. J Psychopharmacol. 2018;32:497–508.
Jelen LA, Lythgoe DJ, Jackson JB, Howard MA, Stone JM, Egerton A. Imaging brain glx dynamics in response to pressure pain stimulation: A (1)H-fMRS Study. Front psychiatry. 2021;12:681419.
Kahn RS, Winter van Rossum I, Leucht S, McGuire P, Lewis SW, Leboyer M, et al. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry. 2018;5:797–807.
Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2016;173:69–77.
Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res. 1997;76:51–61.
Jauhar S, Veronese M, Nour MM, Rogdaki M, Hathway P, Turkheimer FE, et al. Determinants of treatment response in first-episode psychosis: an (18)F-DOPA PET study. Mol Psychiatry. 2019;24:1502–12.
Millgate E, Hide O, Lawrie SM, Murray RM, MacCabe JH, Kravariti E. Neuropsychological differences between treatment-resistant and treatment-responsive schizophrenia: a meta-analysis. Psychological Med. 2022;52:1–13.
Koutsouleris N, Kahn RS, Chekroud AM, Leucht S, Falkai P, Wobrock T, et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. lancet Psychiatry. 2016;3:935–46.
Funding
This research was supported by a grant from the Medical Research Council (MRC) UK, Stratified Medicines Initiative, reference MR/L011794/1 ‘STRATA’. Research at the London site was additionally supported by the Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Center for Mental Health award to South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. J.H.M. has received research funding fromH Lundbeck. O.D.H. is a part-time employee and stockholder of Lundbeck A/s. He has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organised by Angellini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Recordati, Roche and Viatris/ Mylan.
Author information
Authors and Affiliations
Contributions
A.E., J.B.D., R.D., O.D.H., S.L., and J.H.M. conceived and designed the study. K.G., C.C., S.K., S.L., J.L., E. Mikulskaya., E.Millgate., E.O, R.P., N.R., A.S., and K.V.S. acquired the data. L.K. performed project management. A.E. and K.G. performed the analysis. All authors edited or approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
O.D.H. has a patent for the use of dopaminergic imaging. The remaining authors have no disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Egerton, A., Griffiths, K., Casetta, C. et al. Anterior cingulate glutamate metabolites as a predictor of antipsychotic response in first episode psychosis: data from the STRATA collaboration. Neuropsychopharmacol. 48, 567–575 (2023). https://doi.org/10.1038/s41386-022-01508-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01508-w
This article is cited by
-
Glutamatergic basis of antipsychotic response in first-episode psychosis: a dual voxel study of the anterior cingulate cortex
Neuropsychopharmacology (2024)
-
Association between increased anterior cingulate glutamate and psychotic-like experiences, but not autistic traits in healthy volunteers
Scientific Reports (2023)