Abstract
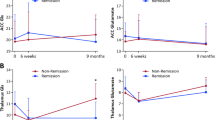
A subgroup of patients with schizophrenia is believed to have aberrant excess of glutamate in the frontal cortex; this subgroup is thought to show poor response to first-line antipsychotic treatments that focus on dopamine blockade. If we can identify this subgroup early in the course of illness, we can reduce the repeated use of first-line antipsychotics and potentially stratify first-episode patients to intervene early with second-line treatments such as clozapine. The use of proton magnetic resonance spectroscopy (1H-MRS) to measure glutamate and Glx (glutamate plus glutamine) may provide a means for such a stratification. We must first establish if there is robust evidence linking elevations in anterior cingulate cortex (ACC) glutamate metabolites to poor response, and determine if the use of antipsychotics worsens the glutamatergic excess in eventual nonresponders. In this study, we estimated glutamate levels at baseline in 42 drug-naive patients with schizophrenia. We then treated them all with risperidone at a standard dose range of 2-6 mg/day and followed them up for 3 months to categorize their response status. We expected to see baseline “hyperglutamatergia” in nonresponders, and expected this to worsen over time at the follow-up. In line with our predictions, nonresponders had higher glutamate than responders, but patients as a group did not differ in glutamate and Glx from the healthy control (HC) group before treatment-onset (F1,79 = 3.20, p = 0.046, partial η2 = 0.075). Glutamatergic metabolites did not change significantly over time in both nonresponders and responders over the 3 months of antipsychotic exposure (F1,31 = 1.26, p = 0.270, partial η2 = 0.039). We conclude that the use of antipsychotics without prior knowledge of later response delays symptom relief in a subgroup of first-episode patients, but does not worsen the glutamatergic excess seen at the baseline. Given the current practice of nonstratified use of antipsychotics, longer-time follow-up MRS studies are required to see if improvement in symptoms accompanies a dynamic shift in glutamate profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Disorders ECPfOPToP: The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. J Clin Psych. 2003;64:2–100.
Dempster K, Li A, Sabesan P, Norman R, Palaniyappan L. Treatment resistance: a time-based approach for early identification in first episode psychosis. J Pers Med. 2021;11:711.
Gudmundson A, Koo A, Virovka A, Amirault A, Soo M, Cho J, et al. In vivo proton MR Spectroscopy of the healthy and diseased human brain. bioRxiv. 2023. https://doi.org/10.1101/2023.02.10.528046.
Nakahara T, Tsugawa S, Noda Y, Ueno F, Honda S, Kinjo M, et al. Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Mol Psych. 2022;27:744–57.
Liemburg E, Sibeijn-Kuiper A, Bais L, Pijnenborg G, Knegtering H, Van der Velde J, et al. Prefrontal NAA and Glx levels in different stages of psychotic disorders: a 3T 1H-MRS study. Sci Rep. 2016;6:21873.
Egerton A, Griffiths K, Casetta C, Deakin B, Drake R, Howes OD, et al. Anterior cingulate glutamate metabolites as a predictor of antipsychotic response in first episode psychosis: data from the STRATA collaboration. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2023;48:567–75.
Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psych. 2014;75:e11–e13.
Iwata Y, Nakajima S, Plitman E, Caravaggio F, Graff-Guerrero A. Glutamatergic neurometabolite levels in patients with ultra treatment-resistant schizophrenia: a cross-sectional 3T Proton MRS study. Biolog Psych. 2018;85:596–605.
Dempster K, Jeon P, MacKinley M, Williamson P, Théberge J, Palaniyappan L. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol Psych. 2020;25:1640–50.
Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naïve patients with psychosis. Psychol Med. 2020;50:2182–93.
Li J, Ren H, He Y, Li ZC, Tang J. Anterior cingulate cortex glutamate levels are related to response to initial antipsychotic treatment in drug-naive first-episode schizophrenia patients. Front Psych. 2020;11:553269.
Kessler RC, Luedtke A. Pragmatic precision psychiatry—a new direction for optimizing treatment selection. JAMA Psych. 2021;78:1384–90.
Hribkova H, Svoboda O, Bartecku E, Zelinkova J, Horinkova J, Lacinova L, et al. Clozapine reverses dysfunction of glutamatergic neurons derived from clozapine-responsive schizophrenia patients. Front Cell Neurosci. 2022;16:830757.
McQueen G, Sendt K-V, Gillespie A, Avila A, Lally J, Vallianatou K, et al. Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophrenia Bull. 2021;47:662–71.
Kantrowitz JT, Grinband J, Goff DC, Lahti AC, Marder SR, Kegeles LS, et al. Proof of mechanism and target engagement of glutamatergic drugs for the treatment of schizophrenia: RCTs of pomaglumetad and TS-134 on ketamine-induced psychotic symptoms and pharmacoBOLD in healthy volunteers. Neuropsychopharmacology. 2020;45:1842–50.
Dogra S, Conn PJ. Metabotropic glutamate receptors as emerging targets for the treatment of schizophrenia. Mol Pharmacol. 2022;101:275–85.
Leucht S, Chaimani A, Krause M, Schneider-Thoma J, Wang D, Dong S, et al. The response of subgroups of patients with schizophrenia to different antipsychotic drugs: a systematic review and meta-analysis. Lancet Psych. 2022;9:84–993.
McCutcheon RA, Pillinger T, Mizuno Y, Montgomery A, Pandian H, Vano L, et al. The efficacy and heterogeneity of antipsychotic response in schizophrenia: a meta-analysis. Mol Psych. 2021;26:1310–20.
McCutcheon RA, Pillinger T, Efthimiou O, Maslej M, Mulsant BH, Young AH, et al. Reappraising the variability of effects of antipsychotic medication in schizophrenia: a meta‐analysis. World Psych. 2022;21:287–94.
Kaminski J, Mascarell-Maricic L, Fukuda Y, Katthagen T, Heinz A, Schlagenhauf F. Glutamate in the dorsolateral prefrontal cortex in patients with schizophrenia: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Biol Psych. 2021;89:270–7.
Merritt K, McCutcheon RA, Aleman A, Ashley S, Beck K, Block W, et al. Variability and magnitude of brain glutamate levels in schizophrenia: a meta and mega-analysis. Mol Psych. 2023:1–10.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psych. 1998;59:22–33.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13:261–76.
Wang Q, Ren H, Li C, Li Z, Li J, Li H, et al. Metabolite differences in the medial prefrontal cortex in schizophrenia patients with and without persistent auditory verbal hallucinations: a 1H MRS study. Transl Psych. 2022;12:116.
Lopez-Persem A, Verhagen L, Amiez C, Petrides M, Sallet J. The human ventromedial prefrontal cortex: sulcal morphology and its influence on functional organization. J Neurosci : Off J Soc Neurosci. 2019;39:3627–39.
Dou W, Palomero-Gallagher N, van Tol M-J, Kaufmann J, Zhong K, Bernstein H-G, et al. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J Neurosci. 2013;33:12698–704.
Wang M, Hu K, Fan L, Yan H, Li P, Jiang T, et al. Predicting treatment response in schizophrenia with magnetic resonance imaging and polygenic risk score. Front Genet. 2022;13:848205.
Leucht S, Davis J, Engel R, Kissling W, Kane J. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scandinavica. 2009;119:7–14.
Elias M, Bloomfield M, Vincent L, Katherine B, Sudhakar S, Naresh R, et al. Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull. 2016;42:744.
Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol. 2015;18:pyu117.
Egerton A, Broberg BV, Van HN, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre 1 H-MRS study (OPTiMiSE). Mol Psych. 2018;23:2145–55.
Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, Mcguire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–21.
Gudmundson AT, Koo A, Virovka A, Amirault AL, Soo M, Cho JH, et al. Meta-analysis and open-source database for in vivo brain Magnetic Resonance spectroscopy in health and disease. Anal Biochem. 2023;676:115227.
Kraguljac NV, Morgan CJ, Reid MA, White DM, Jindal RD, Sivaraman S, et al. A longitudinal magnetic resonance spectroscopy study investigating effects of risperidone in the anterior cingulate cortex and hippocampus in schizophrenia. Schizophrenia Res. 2019;210:239–44.
Reyes-Madrigal F, Guma E, León-Ortiz P, Gómez-Cruz G, Mora-Durán R, Graff-Guerrero A, et al. Striatal glutamate, subcortical structure and clinical response to first-line treatment in first-episode psychosis patients. Prog Neuro-Psychopharmacol Biol Psych. 2022;113:110473.
Merritt K, McGuire PK, Egerton A, Aleman A, Block W, Bloemen OJ, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psych. 2021;78:667–81.
Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psych. 2001;158:1367–77.
Kruse AO, Bustillo JR. Glutamatergic dysfunction in Schizophrenia. Transl Psych. 2022;12:500.
Limongi R, Jeon P, Théberge J, Palaniyappan L. Counteracting effects of glutathione on the glutamate-driven excitation/inhibition imbalance in first-episode schizophrenia: a 7T MRS and dynamic causal modeling study. Antioxidants. 2021;10:75.
Menon V, Palaniyappan L, Supekar K. Integrative brain network and salience models of psychopathology and cognitive dysfunction in schizophrenia. Biol Psych. 2022;94:108–20.
Mouchlianitis ED, Vanes LD, Tracy DK, Fett A-K, Joyce D, Shergill SS. Neuroimaging glutamatergic mechanisms differentiating antipsychotic treatment-response. Sci Rep. 2023;13:8938.
Ouyang X, Pan Y, Chen X, Wu G, Cheng Y, Tan W, et al. Cortical morphological heterogeneity of schizophrenia and its relationship with glutamatergic receptor variations. Eur Psych: J Assoc Eur Psychiatrists. 2023;66:e38.
Liang L, Silva AM, Jeon P, Ford SD, MacKinley M, Théberge J, et al. Widespread cortical thinning, excessive glutamate and impaired linguistic functioning in schizophrenia: A cluster analytic approach. Front Hum Neurosci. 2022;16:954898.
Lutgens D, Joober R, Iyer S, Lepage M, Norman R, Schmitz N, et al. Progress of negative symptoms over the initial 5 years of a first episode of psychosis. Psychol Med. 2019;49:66–74.
Ihler HM, Lyngstad SH, Gardsjord ES, Widing LH, Flaaten CB, Åsbø G, et al. The trajectory of two negative symptom dimensions in first-episode psychosis and the role of cannabis use: A 10-year follow-up study. Schizophrenia Res. 2023;252:317–25.
Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psych. 2013;170:165–72.
Palomero‐Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: Evaluation of the four‐region neurobiological model. Hum Brain Mapp. 2009;30:2336–55.
Pittaluga A, Feligioni M, Longordo F, Luccini E, Raiteri M. Trafficking of presynaptic AMPA receptors mediating neurotransmitter release: neuronal selectivity and relationships with sensitivity to cyclothiazide. Neuropharmacology. 2006;50:286–96.
He J, Wang D, Ban M, Kong L, Xiao Q, Yuan F, et al. Regional metabolic heterogeneity in anterior cingulate cortex in major depressive disorder: A multi-voxel 1H magnetic resonance spectroscopy study. J Affect Disord. 2022;318:263–71.
Jeon P, Limongi R, Ford SD, Mackinley M, Dempster K, Théberge J, et al. Progressive changes in glutamate concentration in early stages of schizophrenia: a longitudinal 7-Tesla MRS study. Schizophrenia Bull Open. 2021;2:072.
Alice E, Akarmi B, Kate M, Grant MQ, Agata S, Philip MG. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal 1H-MRS studies. Front Psych. 2017;8:66.
Kubota M, Moriguchi S, Takahata K, Nakajima S, Horita N. Treatment effects on neurometabolite levels in schizophrenia: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophrenia Res. 2020;222:122–32.
de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psych. 2013;70:1057–66.
Birur B, Kraguljac NV, VerHoef L, Morgan CJ, Jindal RD, Reid MA, et al. Neurometabolic correlates of 6 and 16 weeks of treatment with risperidone in medication-naive first-episode psychosis patients. Transl Psych. 2020;10:15.
Cadena EJ, White DM, Kraguljac NV, Reid MA, Maximo JO, Nelson EA, et al. A longitudinal multimodal neuroimaging study to examine relationships between resting state glutamate and task related BOLD response in schizophrenia. Front Psych. 2018;9:632.
Merritt K, Perez-Iglesias R, Sendt K-V, Goozee R, Jauhar S, Pepper F, et al. Remission from antipsychotic treatment in first episode psychosis related to longitudinal changes in brain glutamate. NPJ Schizophrenia. 2019;5:12.
Szulc A, Galinska B, Tarasow E, Waszkiewicz N, Konarzewska B, Poplawska R, et al. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44:148–57.
Goto N, Yoshimura R, Kakeda S, Nishimura J, Moriya J, Hayashi K, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–22.
Bustillo J, Rowland L, Mullins P, Jung R, Chen H, Qualls C, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psych. 2010;15:629–36.
Aoyama N, Theberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RW, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psych. 2011;198:448–56.
Jauhar S, McCutcheon RA, Veronese M, Borgan F, Nour M, Rogdaki M, et al. The relationship between striatal dopamine and anterior cingulate glutamate in first episode psychosis changes with antipsychotic treatment. Transl Psych. 2023;13:184.
Lavigne KM, Kanagasabai K, Palaniyappan L. Ultra-high field neuroimaging in psychosis: A narrative review. Front Psych. 2022;13:2688.
Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psych. 2007;191:325–34.
Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev. 2015;51:276–95.
Funding
This work was supported by National Key Research and development plan of China (intergovernmental international scientific and technological innovation cooperation project, Grant No:2021YFE0191400), the National Natural Science Foundation of China (NSFC) (Grant No. 81871056 and 82101576), the Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2020zzts287), Science and Technology Innovation Program of Hunan Province (Grant No. 2022RC1040 (ZL)) and China Scholarship Council (Grant No. 202106370192) for LF to train at the Douglas Research Centre. LP acknowledges research support towards this work from the Canada First Research Excellence Fund, awarded to the Healthy Brains, Healthy Lives initiative at McGill University (through New Investigator Supplement to LP); Monique H. Bourgeois Chair in Developmental Disorders and Graham Boeckh Foundation (Douglas Research Centre, McGill University) and a salary award from the Fonds de recherche du Quebec-Sante ́(FRQS).
Author information
Authors and Affiliations
Contributions
XC: Supervision, Funding acquisition, Project administration. LP: Conceptualization, supervision of analysis, writing of original and revised drafts. LF: conceptualization, methodology, acquisition and analysis of data, writing of original and revised drafts. LL: conceptualization, revised drafts. Performed research (acquisition, analysis, and interpretation of data): YW, XM, LY, LO, YH, ZL, CL. All authors contributed to drafting and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
LP reports personal fees for serving as chief editor from the Canadian Medical Association Journals, speaker/consultant fee from Janssen Canada and Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Janssen Canada, Sunovion and Otsuka Canada outside the submitted work. All the other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, L., Liang, L., Wang, Y. et al. Glutamatergic basis of antipsychotic response in first-episode psychosis: a dual voxel study of the anterior cingulate cortex. Neuropsychopharmacol. 49, 845–853 (2024). https://doi.org/10.1038/s41386-023-01741-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01741-x