Abstract
Papillary neoplasms of the breast encompass a wide range of tumor types ranging from the benign intraductal papilloma to in situ and invasive papillary carcinomas. In this review, we considered each tumor entity listed under the Papillary Neoplasms category in the latest WHO Classification of Breast Tumors (5th edition), namely intraductal papilloma, papillary ductal carcinoma in situ, encapsulated papillary carcinoma, solid-papillary carcinoma, and invasive papillary carcinoma. We examined their pathological features, current issues pertaining to diagnosis and prognostication, as well as the latest molecular findings. We also briefly addressed adenomyoepithelioma and the newly included tall cell carcinoma with reversed polarity, highlighting areas where they overlap with papillary neoplasms.
Similar content being viewed by others
Introduction
Papillary neoplasms of the breast encompass a wide range of tumor types ranging from the benign intraductal papilloma (IDP) to in situ and invasive papillary carcinomas. While seemingly unified by a papillary architecture at first glance, a closer inspection of the different types of papillary neoplasms (as defined by the 5th edition of the WHO Classification of Breast Tumors [1]) reveals a fascinating myriad of different morphological and immunohistochemical features that characterize each tumor entity. Over the years, many issues pertaining to the diagnosis and prognostication of papillary tumors have also been studied and debated, some more contentious than others, with each new finding providing increasing clarity as to the nature and biologic behavior of these lesions. Recently, molecular techniques have enabled us to interrogate these tumors at the genomic and transcriptomic level, further adding another dimension to our understanding, although our knowledge of some lesions remains far from complete. In this article, we review pertinent issues encompassing the spectrum of papillary neoplasms of the breast as listed by the WHO classification of tumors (5th edition), namely IDP, papillary ductal carcinoma in situ, encapsulated papillary carcinoma, solid-papillary carcinoma, and invasive papillary carcinoma (IPC). A concise overview of the molecular findings for each entity is included as an attestation to the increasing importance of molecular genetics in surgical pathology. We also briefly address adenomyoepithelioma and the newly included tall cell carcinoma with reversed polarity, highlighting areas where they overlap with papillary neoplasms. Invasive micropapillary carcinoma, which morphologically does not have finger-like fronds with central fibrovascular cores that define the papillary group of neoplasms, will not be the subject of our review.
Intraductal papilloma
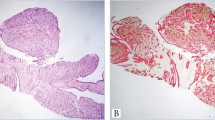
IDP is a benign papillary neoplasm characterized by finger-like fronds with fibrovascular cores lined by a layer of epithelial cells with subjacent myoepithelial cells. It can arise within the large central ducts of the breast in the retroareolar region where it is often solitary (Fig. 1) or within the smaller peripheral ducts where it can be multiple [2,3,4,5]. IDP occurs in women over a wide age range and frequently presents with non-bloody/bloody nipple discharge or as a palpable mass if it has grown to a large size [6]. Small IDPs (microscopic papillomas or micropapillomas) may present as an incidental finding on histology.
On microscopic examination, IDP is composed of arborescent papillary projections with well-developed fibrovascular cores lined by myoepithelial and epithelial cells (Fig. 2). The duct in which it resides is often dilated by the neoplasm and is also lined by myoepithelial cells (Table 1). IDP may be associated with usual type ductal hyperplasia of the epithelial cells, which when florid can appear alarming. However, any concern for an intraductal malignancy can be allayed with the aid of immunohistochemical stains as usual type ductal hyperplasia shows positive staining for high molecular weight cytokeratins and heterogenous/non-uniform staining for estrogen receptor (ER) unlike atypical ductal hyperplasia/in situ carcinoma (Table 2; Figs. 3, 4) [7,8,9]. Apocrine metaplasia, dystrophic microcalcifications and sclerosis are some other changes that can occur. Sclerosis, if extensive, can obliterate the original papillary architecture of the tumor resulting in entrapped ductal elements that are compressed and distorted by fibrosclerotic stroma, mimicking invasive carcinoma [2, 4, 6, 10]. Large papillomas can undergo torsion, infarction and hemorrhage, leading to bloody nipple discharge. Squamous metaplasia may occur subsequently as part of the reparative process. Rare cases with sebaceous metaplasia have also been reported [11].
An intraductal papilloma with characteristic finger-like fronds as well as a component of usual ductal hyperplasia forming a more solid area of proliferation (lower left) (A). Immunohistochemistry for p63/CK14 double stain highlights the myoepithelial cells that are subjacent to the negative staining epithelial cells lining the papillae (B).
Multiple papillomas versus solitary papilloma
It is thought that multiple papillomas of the peripheral ducts confer a greater risk of breast cancer compared to the solitary papilloma of large central ducts. This is due to observations made in early studies of multiple papillomas where they were found to be frequently associated with ductal carcinoma in situ or invasive carcinoma compared to solitary papillomas [3, 12]. In a study by Ali-Fehmi et al. on 61 patients with multiple papillomas (defined as at least 5 papillomas in 2 non-consecutive tissue blocks), 80.4% were associated with at least atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia/lobular carcinoma in situ (ALH/LCIS), either within the papillomas or in the adjacent breast tissue [4]. 54.1% were associated with ductal carcinoma in situ or invasive carcinoma and 21.3% were associated with radial scars. Bilateral disease was seen in 24% of patients, suggesting an increased constitutional risk for breast cancer.
In a larger study of 480 patients of whom 54 had multiple papillomas, it was found that multiple papillomas were associated with a relative risk of breast cancer that is higher than proliferative fibrocystic lesions but lower than ADH/ALH. Solitary papilloma, on the other hand, had a similar risk compared to proliferative fibrocystic lesions. Solitary papilloma with ADH/ALH was associated with a similar risk as ADH/ALH of the breast parenchyma while multiple papillomas with ADH/ALH possessed a higher risk than ADH/ALH of the breast [5]. From the study results, it seems that the papilloma component in a solitary papilloma with ADH/ALH does not further increase the risk already attributed to ADH/ALH while multiple papillomas are associated with an increased risk of breast cancer compared to fibrocystic disease even without atypia. Similar to the study by Ali-Fehmi et al., [4] radial scars were also frequently associated with papillomas [5]. They were significantly more commonly observed in individuals with papillomas compared to those without. Radial scars were also associated with multiple papillomas or solitary papillomas with atypia compared with solitary papillomas without atypia. Sclerosing adenosis and usual ductal hyperplasia were frequently associated with papillomas as a whole [5].
Management of benign papilloma without atypia on core needle biopsy
A key aspect in the clinical management of papillomas is to ascertain the concurrent presence of ADH or in situ carcinoma [13, 14]. Both are part of the same disease process in which there is a clonal proliferation of usually low-grade malignant epithelial cells forming rigid epithelial bridges, arches, cribriform spaces, micropapillary tufts or solid sheets, differentiated only by how extensively it involves the papilloma [5, 10, 15]. As a general rule, a low-grade atypical epithelial proliferation measuring <3 mm in an IDP is considered ADH while that measuring 3 mm or larger is considered ductal carcinoma in situ (DCIS). A 3 mm threshold was first set arbitrarily by Page et al. [10] and while there is limited scientific evidence for this size cut-off, it nonetheless provides a useful guide when we approach low-grade atypical epithelial proliferations in IDP. IDP with an atypical epithelial proliferation of intermediate to high-grade atypia is however considered to have DCIS regardless of the size.
While there is general consensus that IDP with ADH diagnosed on core needle biopsy (CNB) should be excised due to a greater risk of upgrade to malignancy [16], there is some uncertainty as to how to manage papillomas without atypia diagnosed on CNB [17]. This may be partly due to earlier studies reporting higher upgrade rates on excision, raising the concern that these lesions may be undertreated if excision is not performed. Many of these studies, however, had small sample sizes and potential selection bias as pointed out by Jacobs et al. in their review of 5 studies combining a total of 51 benign IDPs [18]. In this group of IDPs, there was an upgrade to DCIS or invasive carcinoma in 11.8% of the cases and an upgrade to ADH/LCIS in 13.7% of cases. Based on these results, the authors felt it may be prudent to excise all papillary lesions, even benign ones, until more data became available.
Subsequent to this review, there were a large number of studies published that examined this issue, with a wide range of upgrade rates ranging from 0% to as high as over 30% [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The reason for such discrepant results between studies can be due to a number of confounding factors as highlighted by Grimm et al. in their review of 60 studies [34]. For example, some studies considered ADH found on excision as an upgrade while others did not. Others analysed papillomas with ADH together with benign papillomas as a group. There were studies which did not exclude cases that showed radiological features discordant from benign IDP seen on CNB. Cases in some reports were not histologically reviewed by a breast pathologist and this may have contributed to differences in upgrade rates as interobserver variability has been found to occur between general pathologists and breast pathologists [35]. Finally, many studies do not comment on whether the malignancy found on excision is related to the benign papilloma seen on CNB or is a separate incidental finding. These may result in artificially elevated upgrade rates. After accounting for these confounders, an examination of the published literature reveals that the upgrade rate (to DCIS or invasive carcinoma) of a benign papilloma on CNB is usually in the range of 5% or less [19,20,21,22, 25,26,27,28,29,30,31] with some studies having slightly higher upgrade rates of 5–10% [23, 24]. Studies with upgrade rates of >10% constitute a minority and may have the aforementioned confounding factors or small sample sizes [32, 33]. Upgrade rates to high risk lesions such as ADH are usually higher, in the range of 10–20% [21, 23]. Grimm et al. [34] in their review of 60 studies with a combined total of 4157 benign IDPs found an upgrade rate to malignancy of 4%. Their own study of 136 benign IDPs had a 0% upgrade to malignancy on excision. In a meta-analysis of 34 studies, Wen and Cheng found an upgrade rate of 7% [16]. As such, in recent years, the authors of most studies have concluded that active surveillance of benign papillomas diagnosed on CNB may be adequate in order to avoid overtreatment. Therapeutic excision via vacuum assisted biopsy followed by active surveillance has also been recommended as viable alternative to open surgery [36, 37].
Molecular genetics of IDP
One of the earlier molecular studies analysing the clonality of IDP found that IDPs were monoclonal proliferations compared to normal breast tissue which was polyclonal, confirming its neoplastic nature [38]. Subsequent loss of heterozygosity (LOH) studies using polymorphic DNA microsatellite markers showed LOH at 16p13 region in 6 of 10 IDPs with florid epithelial hyperplasia, 8 out of 10 IDPs with DCIS and 2 out of 6 papillary carcinomas [39]. Presence of LOH of 16p in both benign IDPs and IDPs with DCIS suggests that the 16p region contains a tumor suppressor gene that is lost early in papillary tumor oncogenesis. This was supported by another study which found LOH at 16p13 and 16q21 in both IDPs as well as malignant papillary tumors (including IDP with ADH or DCIS), suggesting that benign and malignant papillary tumors may share an early common pathway involving 16p13 and 16q21 losses [40]. LOH at 16q23, however, was found only in malignant papillary tumors and not benign IDP, implying that 16q23 losses may be required for progression to a malignant phenotype.
In a subsequent study by Troxell et al. targeted screening for PIK3CA and AKT1 mutations in a group of 61 IDP, 18 IDP with ADH/DCIS/LCIS and 10 papillary carcinomas found that 33% of IDP have PIK3CA mutations (majority exon 20) and another 33% have AKT1 mutations (exon 2) [41]. AKT1 and PIK3CA mutations were mutually exclusive and AKT1 mutations were more commonly seen in papilloma without/mild epithelial hyperplasia while PIK3CA mutations were more commonly observed in papillomas with moderate/florid hyperplasia. Papillomas with ADH/DCIS similarly had a 33% rate of AKT1 mutations (seen in papillomas with ADH and DCIS) while PIK3CA mutations were seen in 28% of cases (only in papillomas with ADH but not DCIS). In contrast, only 1 out of the 10 papillary carcinomas had an AKT1 mutation and this carcinoma contained a focal residual papilloma component. 2 out of 10 papillary carcinomas (20%) displayed PIK3CA mutations (1 encapsulated papillary carcinoma and 1 IPC with tubulopapillary features). The low rate of PIK3CA and AKT1 mutations in papillary carcinomas suggests that papillary carcinomas could have arisen via a different molecular pathway rather than from papillomas with AKT1/PIK3CA mutation. HRAS p.G12D mutation was also found in one papilloma with ADH. In another study of 60 IDPs using digital droplet polymerase chain reaction (PCR), AKT1 and PIK3CA mutations were found similarly in 22.2% and 27.8% of IDPs without atypia respectively. The study also used a fluorescence activated cell sorting technique to perform mutational analysis on luminal and myoepithelial cells separately and found that both luminal and myoepithelial cells showed identical mutations. This suggests that the AKT1 or PIK3CA mutation occurs early in a bipotent progenitor cell which later differentiates into luminal and myoepithelial cells that form the papilloma [42].
In a more recent study comparing 24 IDP without co-existing carcinoma (20 benign IDP and 4 IDP with ADH) against 20 IDP with co-existing DCIS or invasive ductal carcinoma (IDC), IDP without co-existing carcinoma disclosed PIK3CA mutations in 69% of cases and few copy number alterations (CNA), with no significant difference between benign IDP and IDP with ADH [43]. Of 20 IDP (8 benign and 12 atypical papillomas) with co-existing DCIS or IDC (1 with papillary DCIS and invasive mucinous carcinoma, 19 with IDC or DCIS of no special type), 55% (1 benign and 10 atypical papillomas) were found to be clonally related to the malignant component (including the case with papillary DCIS and mucinous carcinoma), with atypical papilloma significantly more likely to be clonally related to the co-existing carcinoma than a benign papilloma with co-existing carcinoma. This suggests that atypical papillomas are more likely to progress to carcinomas compared to benign papillomas, consistent with the higher upgrade rates associated with atypical papillomas on needle biopsies. In contrast to IDPs without co-existing carcinoma, IDPs that were clonally related to co-existing carcinoma also lacked PIK3CA mutations and were enriched for CNA such as 16q loss, 11q loss, and 1q gain. This corroborates the earlier study result by Troxell et al. in which PIK3CA mutations were commonly found in benign IDP and IDP with at most ADH but not in IDP with DCIS or carcinomas arising from IDP [41].
Papillary ductal carcinoma in situ
Papillary DCIS is a subtype of DCIS characterized by an intraductal papillary proliferation with arborizing fibrovascular cores lined by neoplastic epithelial cells but mostly devoid of myoepithelial cells unlike papillomas (Figs. 5–7). Myoepithelial cells, however, are present at the periphery of the duct like other subtypes of DCIS and distinguishes it from encapsulated papillary carcinoma. Papillary DCIS can occur in both central and peripheral ducts and can co-exist with other subtypes of DCIS or encapsulated papillary carcinoma [44]. Apart from a papillary architecture, the neoplastic epithelial cells may also proliferate to form cribriform, solid or micropapillary patterns of growth. A dimorphic morphologic pattern has also been described, in which there is a second population of epithelial cells with abundant clear cytoplasm in addition to the usual neoplastic epithelial cells that line the papillae. These cells have been termed as globoid cells and are not myoepithelial cells [45]. Papillary DCIS must be distinguished from DCIS involving a papilloma. The latter shows evidence of a residual benign papilloma that has been partially effaced by conventional type DCIS process while the former is an intraductal malignant proliferation that recapitulates a papillary architecture [46]. Papillary DCIS shows the same genetic alterations as other subtypes of DCIS of the same nuclear grade.
Encapsulated papillary carcinoma
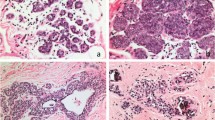
Encapsulated papillary carcinoma (EPC) (also previously termed as intracystic or encysted papillary carcinoma) is an expansile papillary neoplasm that is well circumscribed, within a cystic space, and surrounded by a fibrous wall/capsule (Fig. 8). It usually consists of a single nodule but multinodular cases have been described. A key feature is the absence of myoepithelial cells, both within, as well as at the periphery of the neoplasm [47, 48], differentiating it from papillary DCIS, although both EPC and papillary DCIS can co-exist.
While EPC can occur in patients with a wide age range, it is most common in elderly women who are postmenopausal [49,50,51,52]. It usually presents as a mass that is centrally located and may be associated with nipple discharge and pain [49, 53, 54]. Rarely, it has been reported in males [49, 51, 55, 56]. The papillary fronds in EPC are described as delicate or slender and are classically lined by epithelial cells of low to at most intermediate grade atypia. Mitotic activity is low with one study documenting a mean of 3 mitoses per 10 high power fields (range 0–11) [51]. Areas of cribriform architecture can co-exist and solid areas may even be present focally (Fig. 9). As described earlier, myoepithelial cells are not seen at the epithelial–stroma interface either within the papillary neoplasm or at the periphery, although focal staining for myoepithelial markers may be observed [51]. EPCs with apocrine differentiation have been reported [53, 57]. As expected for the low-grade morphology, a majority of these tumors strongly express ER, PR, and are negative for HER2 on immunohistochemistry and some studies have classified them as luminal A or B tumors based on both immunohistochemical and molecular subtyping [49, 50, 53, 54, 58]. EPCs with apocrine differentiation may be triple negative [57, 59].
In addition to the classic features of EPC described, it is now recognized that a subset of EPCs show high-grade cytologic atypia with a frequency that ranges from 2.5 to 14% depending on the cohorts studied (Figs. 10–12) [51, 52, 60, 61]. However, the incidence is likely low, with one large study of 133 EPCs yielding only 3% of high-grade tumors [52]. Unlike classic EPCs, high-grade EPCs are more likely to be ER negative and thus may show a triple negative phenotype. They are also associated with higher mitotic activity with a series of 12 high-grade EPCs displaying a mean mitotic activity of 22 per 10 high power fields (range 11–32) [62]. High-grade EPCs have also been found to have a higher proportion of associated conventional invasive carcinomas and tend to be larger than EPCs of low to intermediate grade. In a study of 10 patients with high-grade EPC, 1 patient eventually died from the disease. Most of the other patients had a short follow up period of <2 years, leaving the natural course of the disease open to question. This has led the authors of the study to recommend treating high-grade EPCs as invasive carcinomas for now, given the scant data on these tumors. In contrast, the current recommendation for management of conventional low to intermediate grade EPCs is to treat them as in situ lesions [62].
Geographic areas of necrosis are seen in this encapsulated papillary carcinoma which otherwise shows a well-circumscribed, uni-nodular architecture that is typical of encapsulated papillary carcinoma (A). Absence of myoepithelial cells is confirmed on immunohistochemistry for smooth muscle myosin heavy chain (B).
When EPC is associated with a component of invasive carcinoma, only the invasive component is sized and staged accordingly. Invasive carcinoma is defined as the presence of tumor infiltrating beyond the fibrous capsule and must be distinguished from entrapped epithelial elements within the fibrous capsule/areas of sclerosis, not unlike those seen in benign papillomas (Fig. 13) [52]. The invasive tumor can be in the form of invasive carcinoma of no special type, invasive cribriform carcinoma, or invasive mucinous carcinoma [51, 53]. Rarely, the immunohistochemical profile of the invasive carcinoma component (ER/HER2) can differ from the EPC [54]. Invasive carcinoma has also been reported in EPCs with apocrine differentiation [59].
A papillary neoplasm with classic features of an encapsulated papillary carcinoma associated with a focus of tumor extending beyond the fibrous wall of the lesion on the right (A). The tumor is composed of poorly formed tubules and cribriform islands consistent with invasive ductal carcinoma of no special type (B).
Biologic behavior of EPC and the question of whether it is an in situ or invasive carcinoma
When EPC was first widely recognized as a distinct entity, its categorization as an in situ or invasive carcinoma was a subject of much debate. Proponents of EPC being an in situ carcinoma pointed to the very low prevalence of lymph node or distant metastases in pure EPCs without an invasive component. They also suggested (using collagen type IV immunohistochemistry) that the fibrous capsule surrounding EPC may be a form of basement membrane that limits invasion even though myoepithelial cells, which are a hallmark of in situ disease, are absent [60, 63]. Others, however, felt that EPC is more likely to be a form of invasive carcinoma, albeit a more indolent type, based on the fact that EPC does not have a peripheral layer of myoepithelial cells similar to other invasive breast carcinomas and also because pure EPCs have been reported to metastasize to lymph nodes and distant sites even when an invasive carcinoma component is absent [51, 53, 64].
In a review of a number of case series which in total comprised 111 pure EPCs with axillary sampling, 3% were found to have lymph node metastases. 6 out of 266 pure EPCs (2.2%) were also found to have distant metastasis and in some of the cases, the metastatic tumor had a papillary morphology similar to the primary EPC providing support for its metastatic potential [52, 64, 65]. In addition, a subsequent study found staining for collagen type IV to be present even around invasive cribriform carcinoma or around the nests of metastatic carcinoma in axillary lymph nodes, suggesting that type IV collagen immunohistochemistry is not a specific marker of an in situ disease process [51]. Some authors have also proposed that EPC may represent a papillary carcinoma that is transiting from an in situ to an invasive form [47], considering how EPC can be associated with both papillary DCIS and invasive carcinoma. In addition, a study of invasion associated matrix metalloproteinase expression on immunohistochemistry found that EPCs have an expression pattern that is intermediate between that of DCIS and invasive carcinoma [66]. Regardless of its true biological nature, there is general consensus that pure EPC should be staged and managed as an in situ carcinoma (pTis) [46] considering its indolent behavior, in order to avoid overtreatment. Local/regional recurrence after excision has been reported to range from 4.9 to 7% and can be in the form of recurrent pure EPC, IPC or invasive carcinoma of no special type [50, 52].
Solid-papillary carcinoma
Solid-papillary carcinoma (SPC) is defined as a neoplasm with a solid growth pattern with interspersed delicate fibrovascular cores resulting in a solid-papillary architecture. It is most often multinodular, resembling clusters of ducts expanded by a solid-papillary proliferation of cells [67,68,69]. SPC is an uncommon tumor, estimated to form about 1–2% of primary breast cancers in an Asian population [70]. It can occur in a wide age range but the mean/median age of women in most studies is in the elderly postmenopausal woman [67,68,69,70,71,72,73,74]. It usually presents as a mass which can be associated with bloody nipple discharge [67, 68, 74].
Histologically, SPC is composed of expansile solid nodules with interspersed delicate fibrovascular cores that appear to form the scaffold on which a monotonous population of epithelial cells proliferate (Fig. 14). The epithelial cells show round to spindled nuclei of low to at most intermediate grade atypia. High-grade nuclear atypia is rare [69, 73, 75]. Prominent spindling of the epithelial cells can resemble usual type ductal hyperplasia but they usually stain negative for high molecular weight cytokeratins unlike usual ductal hyperplasia (Fig. 15) [76, 77]. The epithelial cells may also have a plasmacytoid or signet ring cell morphology replete with intracytoplasmic mucin [68, 69, 75]. Extracellular mucin, microcystic/microglandular spaces, clusters of foamy macrophages, and microcalcifications may also be present within the solid nodules [69, 75]. Often, the epithelial cells palisade around fibrovascular cores forming rosette-like structures (Fig. 16). Mitotic activity is usually low (<5 mitoses per 10 high power fields) with only occasional cases showing >10 mitoses per 10 high power fields [67, 69, 70, 74]. SPC is considered an in situ disease when the nodules are rounded and well circumscribed with pushing borders without any irregular or jagged contours to suggest an invasive process. Myoepithelial cells may or may not be present at the periphery of these nodules [67, 68, 78]. They are often absent within the nodules but focal staining for myoepithelial cells, especially around the fibrovascular cores have been reported for SPC [14, 72]. Invasive SPC refers to tumors in which the solid nodules are devoid of a myoepithelial cell layer and in addition have irregular, jagged contours associated with a desmoplastic stroma that suggests an invasive process (Fig. 17) [74, 75]. Criteria for distinguishing between in situ and invasive forms of SPC were first suggested in the 4th edition of the WHO Classification of Breast Tumors and were formally defined in the 5th edition. In addition, SPC in situ can also be accompanied by invasive disease, which can be in the form of invasive breast carcinoma of no special type or mucinous, tubular, lobular or mixed subtypes [69, 75]. Conventional type DCIS (either solid, cribriform, micropapillary, or papillary patterns) and benign intraductal papillomas have also been reported to occur in the vicinity of SPC in situ, including cases in which SPC is seen growing into an adjacent benign papilloma [68, 72, 75].
An often multinodular lesion resembling a cluster of ducts that are filled and expanded by a solid-papillary proliferation of tumor cells. The lesion has well-circumscribed, pushing borders without evidence of stromal invasion (A). Closer examination of the nodules shows intervening delicate fibrovascular cores compressed by a solid proliferation of tumor cells of low nuclear grade (B).
Spindling of the tumor cells may occur and may be mistaken for the spindling of cells seen in usual ductal hyperplasia (A). Aggregates of foamy macrophages (center) and microcystic spaces (top and bottom right) can also be present within the tumor nodules (B). Perivascular pseudorosettes may be seen (C).
Invasive solid-papillary carcinoma is diagnosed when the tumor nodules do not have the rounded, pushing borders of in situ disease, but rather, have irregular, jagged contours that may resemble pieces of a jigsaw puzzle when closely apposed, with an infiltrative pattern in the stroma (A). Solid-papillary carcinoma with an invasive carcinoma component, on the other hand, refers to an in situ solid-papillary carcinoma with an area of invasive carcinoma which may be of various histological types including mucinous carcinoma (B; solid-papillary carcinoma component not shown).
On immunohistochemistry, SPC is ER positive, HER2 negative and frequently shows neuroendocrine differentiation [69, 73, 77]. The invasive carcinoma component, if present, can also show neuroendocrine differentiation [70, 75].
Biologic behavior of SPC
Unlike EPC, SPC in situ has not been found to metastasize to regional lymph nodes or distant sites, even in cases where myoepithelial cells are completely absent at the periphery, provided the tumor nodules show well circumscribed, pushing borders without any features of invasive SPC. Tumors with lymph node or distant metastasis are either invasive SPC or SPC with an invasive component [52, 67, 69, 70, 74, 75]. Therefore, even though SPC in situ may lack a myoepithelial cell layer like EPC, there is so far no evidence of metastatic potential even with more than 200 SPC in situ tumors reported in the literature. Local recurrence can occur in both in situ SPC or SPC with invasive disease but the incidence is generally low with 11 cases reported in a review of 253 cases [74]. The same review also documented only 3 deaths, all in SPC with invasive disease, revealing a low-grade behavior. In a separate study, women with SPC in situ were found to have a better disease free survival compared to conventional DCIS [75].
Invasive lobular carcinoma mimicking SPC
Recently, there have been interesting reports of invasive lobular carcinomas with a solid-papillary growth pattern mimicking SPC, although whether these represent the acknowledged solid variant of invasive lobular carcinoma needs clarification. Rakha et al. described 3 such cases where the tumor displayed a solid-papillary architecture replete with interspersed fibrovascular cores but with absent E-cadherin and beta-catenin expression [79]. Focal merging of solid-papillary areas with classic invasive lobular carcinoma at the periphery, coupled with the presence of in situ lobular carcinoma and absent neuroendocrine differentiation supported the diagnosis of an invasive lobular carcinoma rather than SPC or collision tumor. Christgen et al. subsequently documented a similar case and performed gene expression profiling, whole genome copy number profiling and targeted next generation sequencing on both the solid-papillary and classic lobular carcinoma component [80]. They were able to demonstrate that both the solid-papillary and classic lobular components of the tumor shared a common CDH1 mutation and a number of copy number alterations. In addition, the solid-papillary component had additional 20q gain and 1p loss that have been reported to occur in the solid variant of invasive lobular carcinoma. They therefore concluded that both the solid-papillary and classic lobular component of the tumor shared a common clonal ancestry, with the solid-papillary component of the tumor likely arising from classical lobular carcinoma by subclonal evolution.
Molecular genetics of papillary carcinomas (EPC and SPC)
Early LOH studies revealed that encapsulated papillary carcinomas exhibit 16q losses as compared to benign papillomas [81]. In subsequent studies by the same group, they also found LOH at 16p, 17p, 17q, and 18q. c-ERB-B2 amplification and TP53 mutation were absent or rare [82]. Using multicolor fluorescent in situ hybridization (FISH) probes, they also discovered numerical and structural abnormalities of chromosome 1, including fusion of chromosomes 1 and 16 [83].
Using a microarray-based comparative genomic hybridization platform, Duprez et al. tested a group of 63 papillary carcinomas (comprising EPC, SPC, and invasive papillary carcinomas) and compared them against grade- and ER-matched invasive ductal carcinomas of no special type (IDC-NST) [61]. They found that papillary carcinomas as a group showed a similar pattern, albeit lesser number, of gene copy number alterations such as 16q losses, 16p, and 1q gains compared to low-grade, ER-positive IDC-NST. PIK3CA mutations also occurred in 43% of cases, not significantly different from grade- and ER-matched IDC-NSTs. When subjected to unsupervised hierarchical clustering, papillary carcinomas did not form a distinct cluster from grade- and ER-matched IDC-NSTs highlighting their similarity at the genomic level. EPC, SPC, and IPC were also genomically similar to one another, with none forming a distinct cluster when subjected to unsupervised hierarchical clustering. Subsequently, in another study by the same group (Piscuoglio et al.) they found that although papillary carcinomas are genomically similar to IDC-NSTs, they were different at the transcriptomic level with papillary carcinomas having a lower level of expression of genes related to cellular growth, proliferation, cell assembly and organization, cell adhesion, cell movement and migration (including matrix metalloproteinase genes MMP2 and MMP7) [58]. This may explain the indolent behavior, limited invasiveness and favorable prognosis of papillary carcinomas compared to grade- and ER-matched IDC-NSTs. Interestingly, in a prior study by Rakha et al. using immunohistochemistry for matrix metalloproteinases, they similarly found that EPC had a lower level of expression for MMP-2 and MMP-7 when compared to invasive carcinoma. However, EPC demonstrated a higher level of expression for MMP-1 and MMP-9 compared to DCIS and the authors concluded that EPC possessed an expression pattern of matrix metalloproteinases that is intermediate between that of DCIS and invasive carcinoma [66]. In the study by Piscuoglio, it was also found that SPCs had a higher level of expression of genes related to neuroendocrine differentiation when compared to EPC. PAM50 subtyping performed on a small number of cases (4 SPCs and 5 EPCs) classified 4 out of 5 EPCs as luminal A and 1 EPC and all 4 SPCs as luminal B.
In an earlier separate study on the transcriptomic features of mucinous carcinomas and carcinomas with neuroendocrine differentiation of the breast, it was found that SPC was similar to type B (or hypercellular) mucinous carcinoma at the transcriptomic level but was different from grade- and molecular subtype (luminal A or B)-matched IDC-NST [84]. This is unsurprising as it has long been observed that SPC shares a close relationship with hypercellular mucinous carcinoma, with both having common features such as extracellular mucin production and neuroendocrine differentiation as well as frequent co-existence of mucinous carcinoma with SPC. In a study of the relationship between SPC and mucinous carcinoma using immunohistochemistry for WT1 and MUC2, Oh et al. found that SPC with extracellular mucin or concurrent mucinous carcinoma had a higher expression of WT1 and MUC2 on immunohistochemistry compared to those without [73]. SPC and the concurrent mucinous carcinoma also showed good agreement in WT1 with a positive expression tendency while SPC with concurrent non-mucinous invasive carcinoma showed good agreement in WT1 with a negative expression tendency. This suggests that SPC with WT1 expression may go on to develop mucinous carcinoma while SPC without WT1 expression goes on to develop non-mucinous invasive carcinoma.
Invasive papillary carcinoma
IPC is defined as an invasive carcinoma with >90% papillary architecture. Excluding EPC and SPC with stromal invasion as well as invasive SPC, IPC is uncommon. Much of the published literature describing cases of IPC are actually variants of encapsulated or SPC with invasion. As a result, there is not much clinicopathological data on this tumor entity. In accordance with its invasive nature, myoepithelial cells should be absent throughout the tumor. Metastatic tumor to the breast such as ovarian serous carcinoma, lung adenocarcinoma with papillary pattern and thyroid papillary carcinoma should not be mistaken for IPC of the breast. Immunohistochemical markers such as PAX8, WT-1, TTF-1, napsin-A, and thyroglobulin should be able to point out the extramammary origin of the tumor, in conjunction with relevant clinical history.
Adenomyoepithelioma
Adenomyoepithelioma (AME) is a biphasic neoplasm composed of a proliferation of both inner luminal epithelial cells as well as outer myoepithelial cells [85, 86]. It has a variety of architectural patterns including tubular, spindle cell, and lobulated. Papillary architecture has also been described [87, 88]. When a papillary architecture predominates, it can be difficult to differentiate it from IDP with prominent myoepithelial cells. Furthermore, cases of AME with an intraductal growth and merging with intraductal papillomas have also been described [86, 89]. The myoepithelial cells in AME are usually more conspicuous, numerous, and larger than those seen in intraductal papillomas, although it is recognized that there may be significant morphologic overlap (Figs. 18–20). McLaren et al. [88] in their study of 35 AMEs found that 84% of their AMEs had >50% of the lesion composed of myoepithelial cells compared to 31% of papillomas and stressed that the diagnosis of AME should be made only when there is a recognizable proliferation of the myoepithelial cell component. While immunohistochemistry can be used to highlight the prominent myoepithelial cell proliferation in an AME and distinguish them from papillomas, it should be noted that paradoxical staining of high molecular keratins for the luminal cells instead of the myoepithelial cells may be seen [90]. ER expression can be positive or negative in AME. ER-positive AMEs have been found to harbour PIK3CA or AKT1 mutations similar to papillomas but ER-negative AMEs have recurrent HRAS p.Q61 mutations in addition to PIK3CA or PIK3R1 mutations [91]. More recently, one case of ER-positive AME was also found to have a HRAS mutation but it was a p.G12D mutation instead of the p.Q61 mutation that was previously reported [92]. This AME had a multinodular growth pattern and papillary configuration. The p.G12D mutation was also reported in one case of papilloma with ADH in another study, highlighting the overlap between AME and IDP [41]. A RAS Q61R immunohistochemical stain has been shown to decorate 71% of HRAS Q61R mutant AME with 100% specificity, suggesting a potential utility in the diagnostic workup of AME [93]. Malignant AME refers to an AME in which the luminal epithelial component, myoepithelial component or both are malignant [87, 89, 94, 95].
P63 stain of the tumor in Fig. 18 highlights the increased number of myoepithelial cells (A). This tumor was also negative for ER which is unusual for a papilloma. Subsequent resection again showed a papillary tumor (B).
Higher magnification of the resected adenomyoepithelioma in Fig. 19 shows a proliferation of both epithelial and myoepithelial cells (A). The tumor was hypercellular, had increased mitotic activity, extensive areas of necrosis as well as infiltration of the stroma in small nests and singly dispersed cells (B) and was diagnosed as a malignant adenomyoepithelioma.
Tall cell carcinoma with reversed polarity
The morphologic overlap between tall cell carcinoma with reversed polarity and papillary neoplasms is underpinned by its description in the literature by another name: SPC with reverse polarity [96]. This tumor, regarded as invasive, occurs as variably circumscribed, solid nests of cells with central slender fibrovascular cores resulting in a solid-papillary pattern, although true papillae, follicular structures resembling those of the thyroid as well as psammomatous calcifications may also be seen, underscoring its resemblance to papillary thyroid carcinomas [97,98,99,100]. Foamy macrophages are often present within the fibrovascular cores. However, the tumor cells are usually tall columnar in shape and feature abundant eosinophilic cytoplasm, unlike the cells seen in SPC. The nuclei of the cells also display nuclear grooves, occasional intranuclear inclusions, and are polarized away from the base of the cells, the latter giving rise to the appearance of “reversed polarity” (Fig. 21) [97]. Apart from these morphologic features, additional unique characteristics of this tumor that differ from other papillary neoplasms of the breast include a triple negative phenotype (with only a minority of cases staining for hormonal receptors) as well as positive staining for high and low molecular weight cytokeratins CK5/6, CK7, and calretinin [101]. They are also negative for synaptophysin and chromogranin. Unlike thyroid carcinomas, they are negative for TTF-1 and thyroglobulin and stain variably for GATA3, GCDFP15, and mammaglobin. Molecular studies have found recurrent IDH2 p.R172 with concurrent PIK3CA or PIK3R1 mutations in these tumors. An immunohistochemical antibody for IDH2 R172S stains IDH2 mutant tumors in more than 90% of cases and may be a cheaper alternative to IDH2 sequencing in the diagnostic workup of these tumors [101,102,103].
This tumor shows tubulo- to solid-papillary structures lined by columnar cells with vesicular nuclei, small nucleoli and occasional nuclear grooves. The nuclei of many tumor cells are aligned towards the apical/luminal aspect (“reversed polarity”) (A). Immunohistochemistry for IDH2 R172S shows positive staining in tumor cells (B).
Conclusion
Breast papillary neoplasms feature distinct pathological features that allow classification into discrete entities in most cases, despite several morphological similarities that exist on a continuous spectrum ranging from benign to invasive tumors. This is exemplified by EPC that seems to be a tumor in transition from an in situ to invasive carcinoma. Tumors with overlapping features require careful morphologic examination and employment of immunohistochemical stains in order to best categorize them. Cases that defy categorization may represent new entities that have not been described and they warrant reporting and further study, such as the recently recognized tall cell carcinoma with reversed polarity. Newer technologies in the field of molecular genetics may yield further insights that can help improve our diagnosis and prognostication of these tumors.
References
WHO Classification of Tumours Editorial Board. Breast tumours. WHO classification of tumour series, 5th ed., vol. 2. Lyon (France): International Agency for Research on Cancer; 2019.
Murad TM, Contesso G, Mouriesse H. Papillary tumors of large lactiferous ducts. Cancer. 1981;48:122–33.
Ohuchi N, Abe R, Kasai M. Possible cancerous change of intraductal papillomas of the breast. A 3-D reconstruction study of 25 cases. Cancer. 1984;54:605–11.
Ali-Fehmi R, Carolin K, Wallis T, Visscher DW. Clinicopathologic analysis of breast lesions associated with multiple papillomas. Hum Pathol. 2003;34:234–9.
Lewis JT, Hartmann LC, Vierkant RA, Maloney SD, Shane Pankratz V, Allers TM, et al. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am J Surg Pathol. 2006;30:665–72.
Flint A, Oberman HA. Infarction and squamous metaplasia of intraductal papilloma: a benign breast lesion that may simulate carcinoma. Hum Pathol. 1984;15:764–7.
Tan PH, Aw MY, Yip G, Bay BH, Sii LH, Murugaya S, et al. Cytokeratins in papillary lesions of the breast: is there a role in distinguishing intraductal papilloma from papillary ductal carcinoma in situ? Am J Surg Pathol. 2005;29:625–32.
Ichihara S, Fujimoto T, Hashimoto K, Moritani S, Hasegawa M, Yokoi T. Double immunostaining with p63 and high-molecular-weight cytokeratins distinguishes borderline papillary lesions of the breast. Pathol Int. 2007;57:126–32.
Grin A, O’Malley FP, Mulligan AM. Cytokeratin 5 and estrogen receptor immunohistochemistry as a useful adjunct in identifying atypical papillary lesions on breast needle core biopsy. Am J Surg Pathol. 2009;33:1615–23.
Page DL, Salhany KE, Jensen RA, Dupont WD. Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer. 1996;78:258–66.
Jiao YF, Nakamura SI, Oikawa T, Sugai T, Uesugi N. Sebaceous gland metaplasia in intraductal papilloma of the breast. Virchows Arch. 2001;438:505–8.
Carter D. Intraductal papillary tumors of the breast: a study of 78 cases. Cancer. 1977;39:1689–92.
Papotti M, Gugliotta P, Ghiringhello B, Bussolati G. Association of breast carcinoma and multiple intraductal papillomas: an histological and immunohistochemical investigation. Histopathology. 1984;8:963–75.
Moritani S, Ichihara S, Hasegawa M, Endo T, Oiwa M, Shiraiwa M, et al. Uniqueness of ductal carcinoma in situ of the breast concurrent with papilloma: implications from a detailed topographical and histopathological study of 50 cases treated by mastectomy and wide local excision. Histopathology. 2013;63:407–17.
MacGrogan G, Tavassoli FA. Central atypical papillomas of the breast: a clinicopathological study of 119 cases. Virchows Arch. 2003;443:609–17.
Wen X, Cheng W. Nonmalignant breast papillary lesions at core-needle biopsy: a meta-analysis of underestimation and influencing factors. Ann Surg Oncol. 2013;20:94–101.
Liberman L, Bracero N, Vuolo MA, David Dershaw D, Morris EA, Abramson AF, et al. Percutaneous large-core biopsy of papillary breast lesions. AJR Am J Roentgenol. 1999;172:331–7.
Jacobs TW, Connolly JL, Schnitt SJ. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol. 2002;26:1095–110.
Agoff SN, Lawton TJ. Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol. 2004;122:440–3.
Ivan D, Selinko V, Sahin AA, Sneige N, Middleton LP. Accuracy of core needle biopsy diagnosis in assessing papillary breast lesions: histologic predictors of malignancy. Mod Pathol. 2004;17:165–71.
Kiran S, Jeong YJ, Nelson ME, Ring A, Johnson MB, Sheth PA, et al. Are we overtreating intraductal papillomas? J Surg Res. 2018;231:387–94.
Nasehi L, Sturgis CD, Sharma N, Turk P, Calhoun BC. Breast cancer risk associated with benign intraductal papillomas initially diagnosed on core needle biopsy. Clin Breast Cancer. 2018;18:468–73.
Ko D, Kang E, Park SY, Kim SM, Jang M, Yun BL, et al. The management strategy of benign solitary intraductal papilloma on breast core biopsy. Clin Breast Cancer. 2017;17:367–72.
Tseng HS, Chen YL, Chen ST, Wu YC, Kuo SJ, Chen LS, et al. The management of papillary lesion of the breast by core needle biopsy. Eur J Surg Oncol. 2009;35:21–4.
Shiino S, Tsuda H, Yoshida M, Jimbo K, Asaga S, Hojo T, et al. Intraductal papillomas on core biopsy can be upgraded to malignancy on subsequent excisional biopsy regardless of the presence of atypical features. Pathol Int. 2015;65:293–300.
Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006;238:801–8.
Sydnor MK, Wilson JD, Hijaz TA, Massey HD, Shaw De Paredes ES. Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology. 2007;242:58–62.
Rizzo M, Lund MJ, Oprea G, Schniederjan M, Wood WC, Mosunjac M. Surgical follow-up and clinical presentation of 142 breast papillary lesions diagnosed by ultrasound-guided core-needle biopsy. Ann Surg Oncol. 2008;15:1040–7.
Jaffer S, Nagi C, Bleiweiss IJ. Excision is indicated for intraductal papilloma of the breast diagnosed on core needle biopsy. Cancer. 2009;115:2837–43.
Chang JM, Moon WK, Cho N, Han W, Noh DY, Park IA, et al. Risk of carcinoma after subsequent excision of benign papilloma initially diagnosed with an ultrasound (US)-guided 14-gauge core needle biopsy: a prospective observational study. Eur Radiol. 2010;20:1093–100.
Lu Q, Tan EY, Ho B, Chen JJC, Chan PMY. Surgical excision of intraductal breast papilloma diagnosed on core biopsy. ANZ J Surg. 2012;82:168–72.
Tatarian T, Sokas C, Rufail M, Lazar M, Malhotra S, Palazzo JP, et al. Intraductal papilloma with benign pathology on breast core biopsy: to excise or not? Ann Surg Oncol. 2016;23:2501–7.
Pareja F, Corben AD, Brennan SB, Murray MP, Bowser ZL, Jakate K, et al. Breast intraductal papillomas without atypia in radiologic-pathologic concordant core-needle biopsies: rate of upgrade to carcinoma at excision. Cancer. 2016;122:2819–27.
Grimm LJ, Bookhout CE, Bentley RC, Jordan SG, Lawton TJ. Concordant, non-atypical breast papillomas do not require surgical excision: a 10-year multi-institution study and review of the literature. Clin Imaging. 2018;51:180–5.
Rakha EA, Ahmed MA, Ellis IO. Papillary carcinoma of the breast: diagnostic agreement and management implications. Histopathology. 2016;69:862–70.
Rageth CJ, O’Flynn EA, Comstock C, Kurtz C, Kubik R, Madjar H, et al. First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat. 2016;159:203–13.
Rageth CJ, O’Flynn EAM, Pinker K, Kubik-Huch RA, Mundinger A, Decker T, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat. 2019;174:279–96.
Noguchi S, Aihara T, Motomura K, Inaji H, Koyama H. Clonal analysis of human breast tumors by means of polymerase chain reaction. Am J Pathol. 1994;144:1320–5.
Lininger RA, Park WS, Man YG, Pham T, MacGrogan G, Zhuang Z, et al. LOH at 16p13 is a novel chromosomal alteration detected in benign and malignant microdissected papillary neoplasms of the breast. Hum Pathol. 1998;29:1113–8.
Di Cristofano C, Mrad K, Zavaglia K, Bertacca G, Aretini P, Cipollini G, et al. Papillary lesions of the breast: a molecular progression? Breast Cancer Res Treat. 2005;90:71–6.
Troxell ML, Levine J, Beadling C, Warrick A, Dunlap J, Presnell A, et al. High prevalence of PIK3CA/AKT pathway mutations in papillary neoplasms of the breast. Mod Pathol. 2010;23:27–37.
Mishima C, Kagara N, Ikeda J, Morii E, Miyake T, Tanei T, et al. Mutational analysis of AKT1 and PIK3CA in intraductal papillomas of the breast with special reference to cellular components. Am J Pathol. 2018;188:1106–12.
Kader T, Elder K, Zethoven M, Semple T, Hill P, Goode DL, et al. The genetic architecture of breast papillary lesions as a predictor of progression to carcinoma. NPJ Breast Cancer. 2020;6:9.
Perez AA, Balabram D, Salles M, de A, Gobbi H. Ductal carcinoma in situ of the breast: correlation between histopathological features and age of patients. Diagn Pathol. 2014;9:227.
Lefkowitz M, Lefkowitz W, Wargotz ES. Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol. 1994;25:802–9.
Tan PH, Schnitt SJ, van de Vijver MJ, Ellis IO, Lakhani SR. Papillary and neuroendocrine breast lesions: the WHO stance. Histopathology. 2015;66:761–70.
Hill CB, Yeh IT. Myoepithelial cell staining patterns of papillary breast lesions: from intraductal papillomas to invasive papillary carcinomas. Am J Clin Pathol. 2005;123:36–44.
Collins LC, Carlo VP, Hwang H, Barry TS, Gown AM, Schnitt SJ. Intracystic papillary carcinomas of the breast: a reevaluation using a panel of myoepithelial cell markers. Am J Surg Pathol. 2006;30:1002–7.
Zhang J, Zhang T, Wu N, Zhao X, Wang Q, Jiang Y, et al. Intracystic papillary carcinoma of the breast: Experience of a major Chinese cancer center. Pathol Res Pract. 2018;214:579–85.
Wang Y, Lu S, Graves T, Ouseph MM, Resnick MB, Yakirevich E. Can sentinel lymph node biopsy be spared in papillary carcinoma of the breast? Clin Breast Cancer. 2017;17:127–33.
Wynveen CA, Nehhozina T, Akram M, Hassan M, Norton L, Van Zee KJ, et al. Intracystic papillary carcinoma of the breast: An in situ or invasive tumor? Results of immunohistochemical analysis and clinical follow-up. Am J Surg Pathol. 2011;35:1–14.
Rakha EA, Gandhi N, Climent F, Van Deurzen CHM, Haider SA, Dunk L, et al. Encapsulated papillary carcinoma of the breast: an invasive tumor with excellent prognosis. Am J Surg Pathol. 2011;35:1093–103.
Li X, Xu Y, Ye H, Qin S, Hou F, Liu W. Encapsulated papillary carcinoma of the breast: a clinicopathological study of 49 cases. Curr Probl Cancer. 2018;42:291–301.
Steponavičienė L, Gudavičienė D, Briedienė R, Petroška D, Garnelytė A. Diagnosis, treatment, and outcomes of encapsulated papillary carcinoma: a single institution experience. Acta Med Litu. 2018;25:66–75.
Grabowski J, Salzstein SL, Sadler GR, Blair S. Intracystic papillary carcinoma: a review of 917 cases. Cancer. 2008;113:916–20.
Hassan Z, Boulos F, Abbas J, El Charif MH, Assi H, Sbaity E. Intracystic papillary carcinoma: clinical presentation, patterns of practice, and oncological outcomes. Breast Cancer Res Treat. 2020;182:317–23.
Laforga JB, Gasent JM, Sánchez I. Encapsulated apocrine papillary carcinoma of the breast: Case report with clinicopathologic and immunohistochemical study. Diagn Cytopathol. 2011;39:288–93.
Piscuoglio S, Ng CKY, Martelotto LG, Eberle CA, Cowell CF, Natrajan R, et al. Integrative genomic and transcriptomic characterization of papillary carcinomas of the breast. Mol Oncol. 2014;8:1588–602.
Kővári B, Ormándi K, Simonka Z, Vörös A, Cserni G. Apocrine encapsulated papillary carcinoma of the breast: the first reported case with an infiltrative component. J Breast Cancer. 2018;21:227–30.
Esposito NN, Dabbs DJ, Bhargava R. Are encapsulated papillary carcinomas of the breast in situ or invasive? A basement membrane study of 27 cases. Am J Clin Pathol. 2009;131:228–42.
Duprez R, Wilkerson PM, Lacroix-triki M, Lambros MB, MacKay A, A’Hern R, et al. Immunophenotypic and genomic characterisation of papillary carcinomas of the breast. J Pathol. 2012;226:427–41.
Rakha EA, Varga Z, Elsheik S, Ellis IO. High-grade encapsulated papillary carcinoma of the breast: an under-recognized entity. Histopathology. 2015;66:740–6.
Bhargava R, Esposito NN, Dabbs DJ. Intracystic papillary carcinomas of the breast are more similar to in situ carcinomas than to invasive carcinoma. Am J Surg Pathol. 2011;35:778–9.
Brogi E. In response. Am J Surg Pathol. 2011;35:779–81.
Fayanju OM, Ritter J, Gillanders WE, Eberlein TJ, Dietz JR, Aft R, et al. Therapeutic management of intracystic papillary carcinoma of the breast: the roles of radiation and endocrine therapy. Am J Surg. 2007;194:497–500.
Rakha EA, Tun M, Junainah E, Ellis IO, Green A. Encapsulated papillary carcinoma of the breast: a study of invasion associated markers. J Clin Pathol. 2012;65:710–4.
Maluf HM, Koerner FC. Solid papillary carcinoma of the breast: a form of intraductal carcinoma with endocrine differentiation frequently associated with mucinous carcinoma. Am J Surg Pathol. 1995;19:1237–44.
Tsang WYW, Chan JKC. Endocrine ductal carcinoma in situ (E-DCIS) of the breast: A form of low-grade DCIS with distinctive clinicopathologic and biologic characteristics. Am J Surg Pathol. 1996;20:921–43.
Nassar H, Qureshi H, Adsay NV, Visscher D. Clinicopathologic analysis of solid papillary carcinoma of the breast and associated invasive carcinomas. Am J Surg Pathol. 2006;30:501–7.
Otsuki Y, Yamada M, Shimizu SI, Suwa K, Yoshida M, Tanioka F, et al. Solid-papillary carcinoma of the breast: Clinicopathological study of 20 cases. Pathol Int. 2007;57:421–9.
Nicolas MM, Wu Y, Middleton LP, Gilcrease MZ. Loss of myoepithelium is variable in solid papillary carcinoma of the breast. Histopathology. 2007;51:657–65.
Moritani S, Ichihara S, Kushima R, Okabe H, Bamba M, Kobayashi TK, et al. Myoepithelial cells in solid variant of intraductal papillary carcinoma of the breast: a potential diagnostic pitfall and a proposal of an immunohistochemical panel in the differential diagnosis with intraductal papilloma with usual ductal hyperplasia. Virchows Arch. 2007;450:539–47.
Oh EJ, Koo JS, Kim JY, Jung WH. Correlation between solid papillary carcinoma and associated invasive carcinoma according to expression of WT1 and several MUCs. Pathol Res Pract. 2014;210:953–8.
Guo S, Wang Y, Rohr J, Fan C, Li Q, Li X, et al. Solid papillary carcinoma of the breast: a special entity needs to be distinguished from conventional invasive carcinoma avoiding over-treatment. Breast. 2016;26:67–72.
Tan BY, Thike AA, Ellis IO, Tan PH. Clinicopathologic characteristics of solid papillary carcinoma of the breast. Am J Surg Pathol. 2016;40:1334–42.
Rabban JT, Koerner FC, Lerwill MF. Solid papillary ductal carcinoma in situ versus usual ductal hyperplasia in the breast: a potentially difficult distinction resolved by cytokeratin 5/6. Hum Pathol. 2006;37:787–93.
Maeda I, Tajima S, Kanemaki Y, Tsugawa K, Takagi M. Use of immunohistochemical analysis of CK5/6, CK14, and CK34betaE12 in the differential diagnosis of solid papillary carcinoma in situ from intraductal papilloma with usual ductal hyperplasia of the breast. SAGE Open Med. 2018;6:205031211881154.
Dickersin GR, Maluf HM, Koerner FC. Solid papillary carcinoma of breast: an ultrastructural study. Ultrastruct Pathol. 1997;21:153–61.
Rakha EA, Abbas A, Sheeran R. Invasive lobular carcinoma mimicking papillary carcinoma: a report of three cases. Pathobiology. 2016;83:221–7.
Christgen M, Bartels S, van Luttikhuizen JL, Schieck M, Pertschy S, Kundu S, et al. Subclonal analysis in a lobular breast cancer with classical and solid growth pattern mimicking a solid-papillary carcinoma. J Pathol Clin Res. 2017;3:191–202.
Tsuda H, Uei Y, Fukutomi T, Hirohashi S. Different incidence of loss of heterozygosity on chromosome 16q between intraductal papilloma and intracystic papillary carcinoma of the breast. Jpn J Cancer Res. 1994;85:992–6.
Tsuda H, Fukutomi T, Hirohashi S. Pattern of gene alterations in intraductal breast neoplasms associated with histological type and grade. Clin Cancer Res. 1995;1:261–7.
Tsuda H, Takarabe T, Susumu N, Inazawa J, Okada S, Hirohashi S. Detection of numerical and structural alterations and fusion of chromosomes 16 and 1 in low-grade papillary breast carcinoma by fluorescence in situ hybridization. Am J Pathol. 1997;151:1027–34.
Weigelt B, Geyer FC, Horlings HM, Kreike B, Halfwerk H, Reis-Filho JS. Mucinous and neuroendocrine breast carcinomas are transcriptionally distinct from invasive ductal carcinomas of no special type. Mod Pathol. 2009;22:1401–14.
Zarbo RJ, Oberman HA. Cellular adenomyoepithelioma of the breast. Am J Surg Pathol. 1983;7:863–70.
Rosen PP. Adenomyoepithelioma of the breast. Hum Pathol. 1987;18:1232–7.
Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991;15:554–68.
McLaren BK, Smith J, Schuyler PA, Dupont WD, Page DL. Adenomyoepithelioma: clinical, histologic, and immunohistologic evaluation of a series of related lesions. Am J Surg Pathol. 2005;29:1294–9.
Loose JH, Patchefsky AS, Hollander IJ, Lavin LS, Cooper HS, Katz SM. Adenomyoepithelioma of the breast. A spectrum of biologic behavior. Am J Surg Pathol. 1992;16:868–76.
Moritani S, Ichihara S, Yatabe Y, Hasegawa M, Iwakoshi A, Hosoda W, et al. Immunohistochemical expression of myoepithelial markers in adenomyoepithelioma of the breast: a unique paradoxical staining pattern of high-molecular weight cytokeratins. Virchows Arch. 2015;466:191–8.
Geyer FC, Li A, Papanastasiou AD, Smith A, Selenica P, Burke KA, et al. Recurrent hotspot mutations in HRAS Q61 and PI3K-AKT pathway genes as drivers of breast adenomyoepitheliomas. Nat Commun. 2018;9:1816.
Ginter PS, McIntire PJ, Kurtis B, Mirabelli S, Motanagh S, Hoda S, et al. Adenomyoepithelial tumors of the breast: molecular underpinnings of a rare entity. Mod Pathol. 2020;33:1764–72.
Pareja F, Toss MS, Geyer FC, da Silva EM, Vahdatinia M, Sebastiao APM, et al. Immunohistochemical assessment of HRAS Q61R mutations in breast adenomyoepitheliomas. Histopathology. 2020;76:865–74.
Michal M, Baumruk L, Burgert J, Manhalova M. Adenomyoepithelioma of the breast with undifferentiated carcinoma component. Histopathology. 1994;24:274–6.
Rasbridge SA, Millis RR. Adenomyoepithelioma of the breast with malignant features. Virchows Arch. 1998;432:123–30.
Foschini MP, Asioli S, Foreid S, Cserni G, Ellis IO, Eusebi V, et al. Solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms. Am J Surg Pathol. 2017;41:887–95.
Eusebi V, Damiani S, Ellis IO, Azzopardi JG, Rosai J. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: report of 5 cases. Am J Surg Pathol. 2003;27:1114–8.
Cameselle-Teijeiro J, Abdulkader I, Barreiro-Morandeira F, Ruiz-Ponte C, Reyes-Santías R, Chavez E, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: a case report. Int J Surg Pathol. 2006;14:79–84.
Tosi AL, Ragazzi M, Asioli S, Del Vecchio M, Cavalieri M, Eusebi LHU, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: report of 4 cases with evidence of malignant potential. Int J Surg Pathol. 2007;15:14–9.
Chang SY, Fleiszer DM, Mesurolle B, Khoury M EL, Omeroglu A. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma. Breast J. 2009;15:531–5.
Alsadoun N, MacGrogan G, Truntzer C, Lacroix-Triki M, Bedgedjian I, Koeb MH, et al. Solid papillary carcinoma with reverse polarity of the breast harbors specific morphologic, immunohistochemical and molecular profile in comparison with other benign or malignant papillary lesions of the breast: a comparative study of 9 additional cases. Mod Pathol. 2018;31:1367–80.
Chiang S, Weigelt B, Wen HC, Pareja F, Raghavendra A, Martelotto LG, et al. IDH2 mutations define a unique subtype of breast cancer with altered nuclear polarity. Cancer Res. 2016;76:7118–29.
Pareja F, da Silva EM, Frosina D, Geyer FC, Lozada JR, Basili T, et al. Immunohistochemical analysis of IDH2 R172 hotspot mutations in breast papillary neoplasms: applications in the diagnosis of tall cell carcinoma with reverse polarity. Mod Pathol. 2020;33:1056–64.
Acknowledgements
The authors would like to thank Dr. Wentao Yang, Department of Pathology, Fudan University Shanghai Cancer Center for Fig. 21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tay, T.K.Y., Tan, P.H. Papillary neoplasms of the breast—reviewing the spectrum. Mod Pathol 34, 1044–1061 (2021). https://doi.org/10.1038/s41379-020-00732-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-00732-3
This article is cited by
-
Mammary mucinous cystadenocarcinoma with long-term follow-up: molecular information and literature review
Diagnostic Pathology (2023)
-
Breast carcinomas of low malignant potential
Virchows Archiv (2022)
-
Papillary lesions of the breast
Virchows Archiv (2022)