Abstract
Introduction:
A lyophilized formulation of lucinactant has been developed to simplify preparation and dosing. Endotracheal administration of surfactant can be associated with potentially harmful transient hemodynamic changes including decreases in cerebral blood flow and delivery of O2 to the brain. Efficacy and peri-dosing effects of poractant alfa and a lyophilized form of lucinactant were compared in this study.
Methods:
Premature lambs (126–129 d gestation) were delivered by c-section, tracheostomized, ventilated, and instrumented with cerebral laser Doppler flowmetry and tissue PO2 probes. Pulmonary compliance and tidal volumes were monitored continuously and surfactant lung distribution was assessed. Lambs received either poractant alfa or lyophilized lucinactant and were monitored for 3 h after treatment.
Results:
Both groups showed significant improvements in arterial pCO2, pH, pulmonary compliance, and tidal volume (all P < 0.01), a similar intra-pulmonary distribution profile, and no significant changes in arterial blood pressure or cerebral blood flow. Administration of poractant alfa was associated with higher mean airway pressures from 75 min post-dosing and transiently decreased heart rate and increased brain tissue PO2 during the first 30 min after treatment.
Discussion:
In this newborn lamb model of respiratory distress, lyophilized lucinactant results in improved lung function as compared with poractant alfa.
Similar content being viewed by others
Main
The biophysical characteristics of pulmonary surfactant were first described by Clements in the 1950s (1), and insufficient amounts of surfactant in the lung extracts of infants who died from hyaline membrane disease were documented by Avery and Mead (2). After the initial report on clinical effectiveness of exogenous, bovine-derived surfactant by Fujiwara et al. (3), a pilot clinical trial of human surfactant for neonatal respiratory distress syndrome (RDS) was initiated in 1981 (4), followed by studies of bovine lung extracts (5,6), thus opening the era of surfactant replacement therapy for the treatment and prophylaxis of neonatal RDS.
Currently, several therapeutic surfactants with different compositions are commercially available for the management of neonatal RDS. All of these surfactants, including poractant alfa, beractant, calfactant, and surfactant-TA, are derived from animals. These products contain both hydrophobic surfactant proteins (SPs) B and C in various quantities and proportions. The critical importance of SP-B was demonstrated by Clark et al., who showed respiratory failure occurring soon after birth in an SP-B gene knockout mouse model (7). Synthetic surfactants such as colfosceril palmitate and pumactant did not contain any SPs (8) and are no longer used for the treatment of RDS. A meta-analysis by the Cochrane Library showed that animal-derived surfactants, when used to treat RDS, provide a significant benefit over synthetic surfactants by decreasing the incidences of pulmonary air leaks and all-cause mortality (9). Most of the animal-derived surfactants that are currently available require special handling, including refrigeration during storage and an appropriate warming procedure before use, resulting in a potentially longer preparation time for administration.
One of the most common clinically used surfactant preparations is poractant alfa (Curosurf), a porcine-derived preparation that has been shown to be effective as compared with beractant in multiple clinical trials and was noted to be superior to beractant by Ramanathan et al. (10). Surfaxin (lucinactant; sinapultide surfactant), an FDA approved, synthetic surfactant that contains a peptide, sinapultide, combined in an aqueous dispersion with the phospholipids dipalmityolophosphatidylocholine and phosphatidylglycerol and palmitic acid (11). Sinapultide is a 21-amino-acid peptide composed of lysine and leucine based on the residues 64–79 derived from SP-B (12). A phase III clinical study and a long-term follow-up demonstrated that this liquid synthetic surfactant is superior in RDS-related mortality and 1-y survival as compared with the synthetic lyophilized colfosceril palmitate (13,14). A lyophilized dosage form of lucinactant has been developed recently, but has not been tested against an approved formulation for surfactant replacement therapy. Previously, only lyophilized colfosceril palmitate was tested against nonlyophilized surfactant preparations, but this preparation lacked SPs and is no longer commercially available.
The aim of this study was to assess the biological response of a new lyophilized formulation of lucinactant in an in vivo animal model of respiratory distress in comparison with poractant alfa, the most commonly used clinical surfactant preparation. Using an established premature lamb model of neonatal respiratory failure (15,16), we tested the hypothesis that the two surfactant formulations would result in comparable improvements in pulmonary function and peri-dosing events. We also tested the hypothesis that the two surfactants would be distributed differently within the lungs. The selected surfactant preparations were evaluated on the basis of biological response (PaO2, PCO2, respiratory system compliance (CRS), and mean airway pressure), peri-dosing changes in cerebral blood flow (CBF), brain tissue oxygen tensions, mean arterial blood pressure and heart rate, and pulmonary distribution.
Results
The mean weight ± SEM of the poractant alfa animals was 2.6 ± 0.2 kg, and the mean weight of the lyophilized lucinactant animals was 2.7 ± 0.1 kg.
Blood Gas Measurement
All lambs developed respiratory and metabolic acidosis and were treated as described in the Methods section. After the surfactant instillation, arterial PO2, PCO2, and pH improved significantly in lambs in both treatment groups. Arterial PO2 tended to increase faster after the poractant alfa treatment, but the difference between groups was not significant (ANOVA). Arterial PCO2 decreased more rapidly and was significantly lower at the 15-min time point (P < 0.01) in lambs treated with poractant alfa as compared with lambs treated with lyophilized lucinactant ( Figure 1 ).
Three-hour time course of blood gas and pH values after tracheal instillation of poractant alfa (white circles, n = 6) and lyophilized lucinactant (black circles, n = 6) given to fetal sheep delivered at 85% gestation. Note resolving metabolic and respiratory acidosis for both surfactants. * = Statistical difference between surfactant groups (P < 0.01, two-way ANOVA with Bonferroni post-test for arterial PCO2) at the 15-min time point. No other statistical differences between surfactant preparations were observed (P < 0.05). † = Significant difference from baseline levels (one-way ANOVA with Bonferroni post-test, P < 0.05).
Pulmonary Function
Pulmonary compliance and tidal volume increased significantly after the administration of surfactant as compared with baseline measurements in both treatment groups (one-way ANOVA, P < 0.01), but there were no differences in compliance or tidal volumes between treatment groups. However, mean airway pressure was significantly lower from 75 min post-treatment to the end of the observation period in lambs treated with lyophilized lucinactant as compared with lambs treated with poractant alfa (two-way ANOVA, P < 0.01) ( Figure 2 ).
Time course of respiratory parameters after instillation of poractant alfa (white circles, n = 6) and lyophilized lucinactant (black circles, n = 6) to prematurely delivered lambs. Note two-fold increase in dynamic compliance for both surfactants. * = Statistical difference between surfactant groups (two-way ANOVA with Bonferroni post-test, P < 0.01). † = Significant difference from baseline levels (one-way ANOVA with Bonferroni post-test, P < 0.05).
CBF, Brain Tissue PO2, Arterial Blood Pressure, and Heart Rate
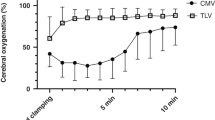
Compared with lyophilized lucinactant, treatment with poractant alfa resulted in a significant increase in brain tissue PO2 (P < 0.05) that persisted for 5 min after the administration of surfactant and was observed again during the third hour of the experiment. There was also a significant decrease in mean heart rate from 188 ± 6 to 160 ± 11 beats per minute for nearly 20 min after poractant alfa administration (two-way ANOVA, P < 0.05) as compared with treatment with lyophilized lucinactant. There was no significant difference in mean arterial blood pressure or CBF between the treatment groups or relative to the baseline period ( Figure 3 ).
Peri-dosing events after instillation of poractant alfa (white circles, n = 6) and lyophilized lucinactant (black circles, n = 6) to prematurely delivered lambs expressed as changes in cerebral blood flow, brain tissue O2, mean arterial blood pressure, and heart rate (bpm, beats per min). * = Statistical difference between surfactant groups (two-way ANOVA with Bonferroni post-test, P < 0.01). † = Significant difference from baseline levels (one-way ANOVA with Bonferroni post-test, P < 0.05).
Distribution of Surfactant
The surfactant distribution data comprise the results of 360 samples taken from five lungs in each group. The average weight of individual tissue samples was 0.89 ± 0.03 g in the animals given lyophilized lucinactant and 0.92 ± 0.04 g in lambs given poractant alfa. An average of 25,554 ± 1,863 microspheres and 26,529 ± 2,212 microspheres were counted in the individual pieces of lyophilized lucinactant and poractant alfa lungs, respectively, numbers that enable surfactant distribution measurements with >3% precision 95% of the time (17). No significant differences in distribution of surfactant within the lung were found between lucinactant and poractant alfa ( Figure 4 ).
Pulmonary distribution of instilled surfactants. (a) Percentage of lung tissue containing different relative amounts of microspheres mixed with instilled surfactant. Scatter plots depict counts of labeled microspheres in lung pieces. The distribution after the instillation of lyophilized lucinactant (black circles, n = 6) is not different than after instillation of poractant alfa (white circles, n = 6). (b) Microsphere counts for lyophilized lucinactant (black bars, solid line) and poractant alfa (white bars, dashed line) expressed as deviation from a homogenous distribution based on wet lung weight, with “0” as an ideal distribution. med, medial aspect of the lung lobe; mid, middle aspect of the lung lobe; lat, lateral aspect of the lung lobe.
Discussion
This initial evaluation of lyophilized lucinactant compared with poractant alfa is an important comparison because poractant alfa is one of the most widely used exogenous surfactants for management of neonatal RDS throughout the world. In this study, the biological response observed after treatment with poractant alfa and lyophilized lucinactant demonstrated improvement compared with baseline, but was similar between groups through 3 h after the administration of surfactant. Pulmonary compliance, tidal volume, and arterial PO2 levels were the same in both groups from 90 to 180 min after the administration of surfactant; however, mean airway pressure was significantly lower in the lyophilized lucinactant group vs. the poractant alfa group. Poractant alfa resulted in a more rapid decrease in arterial PCO2 by 15 minutes post dose, and a significant increase of brain tissue O2 at 5 min and 3 h post-dosing as compared with lyophilized lucinactant. There were no significant differences in lung distribution of the two surfactants.
This is the first in vivo study report demonstrating that lyophilized lucinactant improves lung function indicated by an increase in pulmonary compliance and tidal volume, and a decrease in mean airway pressure. The observed improvements in lung function are comparable with what was previously reported following administration of nonlyophilized lucinactant in liquid instillate form (15). Previously, Vidyasagar et al. showed that lyophilized surfactant-TA, a bovine-derived exogenous surfactant, when given to baboons 2 h after delivery, showed a beneficial effect on the arterial-to-alveolar PaO2 ratio and pulmonary compliance and reduced respiratory support as compared with no treatment (18). These findings support the concept that lyophilized preparations are effective alternatives to liquid instillate surfactant formulations. Cummings et al. compared four different surfactants, including a lyophilized surfactant, colfosceril palmitate, in a 126-d-old premature newborn lamb model. Except for colfosceril palmitate, all the surfactants tested improved mortality, arterial oxygenation, and pulmonary compliance at 24 h post delivery (19). Although surfactant-TA and colfosceril palmitate are lyophilized preparations, each showed different biological responses in their respective studies. The positive effect of surfactant-TA may have been linked to the function of its SP-B component, which is absent in colfosceril palmitate. Lyophilized lucinactant more closely resembles surfactant-TA in that these lyophilized surfactants contain either SP-B or a peptide mimic of SP-B.
Despite comparable tidal volumes, arterial PCO2 was significantly higher in the lucinactant lambs at 15 min after the administration of surfactant, suggesting transiently decreased ventilation/perfusion matching compared with the poractant alfa animals. Apart from transiently lower PCO2 in the poractant alfa group, significant differences in blood gas and pulmonary function values between the two treatment groups beyond 30 min post-dosing were not observed. Nevertheless, the animals treated with lyophilized lucinactant required significantly lower mean airway pressures after 75 min in order to maintain protocol-required tidal volume. Lower mean airway pressure may be linked to study design or drug response. This may have been related to a number of variables known to affect the distribution and function of surfactant that were not similar between the two surfactant preparations studied. For example, the rheology of aggregated dispersions and suspensions of surfactant varies significantly with differences in phospholipid concentrations, which are nearly three-fold more concentrated in poractant alfa (80 mg/ml) compared with lucinactant (30 mg/ml). The volume of instillate (which was nearly three-fold greater in the lucinactant group) is also directly related to the distribution of surfactant to the distal airways (20,21). Pulmonary distribution also varies in direct proportion to the viscosity of the surfactant (22,23,24), and although a quantitative comparison of viscocities of the two formulations has not been carried out, it is likely another variable that differs between the two groups.
Although the goals of this study precluded controlling for variables known to affect pulmonary distribution of surfactant, the differences in gross distribution within the lungs were not measured. This finding is in contrast to a previous study in which liquid lucinactant was more homogeneously distributed than poractant alfa (15), and suggests that the distribution characteristics of lyophilized lucinactant may be altered from that of the parent liquid form. It is worth noting that the measurements of comparable pulmonary distributions on a macroscopic scale do not rule out the possibility of differing distribution of the surfactants within the airways. Such differences, together with the differing physical and chemical characteristics of the two formulations, may also have contributed to the lower mean airway pressures required in the lambs treated with lucinactant.
Analysis of peri-dosing changes in brain oxygenation and heart rate revealed some differences between the two surfactant preparations. Poractant alfa caused a rapid increase in brain tissue PO2 to levels significantly greater than either those of the fetal sheep in utero (25) or adult animals breathing 21% oxygen (26). Both the present and previous studies (15) have shown significantly higher brain tissue PO2 levels and decreasing heart rate after the administration of poractant alfa. It is possible that the decrease in heart rate is related to a chemoreceptor-mediated bradycardia in response to increased PO2, a mechanism that has been described in adults (27) as well as newborn infants (28). The cause of the differences in brain tissue PO2 between the two groups, particularly after 3 h, when arterial PO2 and CBF are similar, needs further evaluation.
There was a difference in the logistics of surfactant preparation before administration. Lyophilized lucinactant was reconstituted in sterile water, which took approximately 30 s, shaken for 10 s, and then drawn into a syringe, whereas poractant alfa was warmed to room temperature with the vial gently turned upside down for 10 min for uniform suspension and then drawn into a syringe. Although we compensated for these differences in our study so that the administration of surfactant occurred at the same time relative to birth for both types of surfactant, in a clinical setting, shorter preparation time and drug readiness at any time and place, without influencing the biological response of the drug, might be an important variable under certain circumstances.
In summary, we observed comparable improvements in blood gases and pulmonary function at 3 h after lyophilized lucinactant and poractant alfa administration in a premature lamb model of respiratory failure. However, lyophilized lucinactant required lower mean airway pressures to achieve similar tidal volumes as compared with poractant alfa. The higher volume of lyophilized lucinactant administered to lambs was not associated with an increase in observed peri-dosing adverse effects. Potential additional benefits related to improved logistics of drug handling and shorter preparation time as well as the physical profile of the new lyophilized surfactant should be evaluated in future research.
Methods
Surgical Instrumentation
Animal care protocols were pre-approved by the Loma Linda University Institutional Animal Care and Use Committee. Pregnant ewes were obtained from Nebeker Ranch (Lancaster, CA) at 124–126 d gestation (term ~147 d). Ewes were carrying either singletons or twins. In the case of twins, only one of the lambs was studied. At 48 and 24 h before delivery of the lambs, the ewes were treated with 12 mg of betamethasone intramuscularly to enhance fetal lung maturity, a procedure chosen to mimic obstetrical management of pregnant women with anticipated preterm delivery. The ewes were given 500 mg of intravenous thiopental and then intubated and ventilated with 2% isoflurane. Amniotic fluid was aspirated before exposing the fetal head, which was exteriorized through a midline incision in the abdomen of the ewe. A 4.0-mm (outer diameter) endotracheal tube (Mallinckrodt, St. Louis, MO) was inserted up to 7 cm through a tracheotomy at the second tracheal ring, locating the tip approximately 1 cm above the carina. [The endotracheal tube was clamped on insertion to prevent the loss of lung fluid. Each lamb was instrumented bilaterally with a composite laser Doppler flowmetry and brain tissue oxygen tensions probe (diameter ~400 μm, Oxyford Optronix Ltd, Oxford, UK) inserted through a burr hole drilled on either side of the skull, approximately 1 cm posterior to the coronal suture and 0.5 cm lateral to the sagittal suture as previously described (25). The tip of the probe was inserted into the parietal cortex approximately 0.5 cm below the dura, a position previously determined to position the probe tip in the grey matter (25). After surgical instrumentation, the lamb was delivered and weighed, the umbilical cord ligated and severed, amniotic fluid was suctioned out of the airway using a 15-cm catheter, and mechanical ventilation was initiated. Catheters were inserted into an umbilical artery and vein for measurement of arterial blood gases, blood pressure monitoring, and administration of medications. The lambs received an intravenous infusion of 5% glucose in water at 6 ml/h, 1 mg/kg/h of ketamine, and 0.1 mg/kg/h of pancuronium throughout the experiment.
Ventilation
Mechanical ventilation was initiated at 60 breaths per minute, peak inspiratory pressure of 25 cm H2O, positive end expiratory pressure of 5 cm H2O, and an inspiratory/expiratory ratio of 1:2. Fractional inspired oxygen was held at 1.0 for the duration of the study. Before the administration of surfactant, peak inspiratory pressure was increased as needed in order to maintain tidal volume between 3 and 5 ml/kg. After the administration of surfactant, peak inspiratory pressure was primarily adjusted to achieve tidal volumes of 6–8 ml/kg, resulting in PCO2 of 40–60 torr, and pH of 7.25–7.5. Metabolic acidosis was treated by intravascular volume expansion with 10 ml/kg normal saline and administration of intravenous tromethamine 30 mEq/100 ml at 3 ml/kg for a base excess less than −7 mEq/l.
Protocol Timeline
A total of 12 premature lambs were studied; six received poractant alfa and six received lyophilized lucinactant. Surfactant was administered by bolus into the endotracheal tube after a 20-min period of baseline measurements. The lambs were monitored for a total of 180 min from the time of initiating surfactant administration.
Administration of Surfactant
Poractant alfa (Curosurf, Chiesi Farmaceutici, Parma, Italy) was administered consistent with its prescribing information. Vials were first warmed to room temperature over a period of 5–10 min and then the appropriate dose (based on 2.5 ml/kg and 200 mg/kg phospholipids) was drawn into a 5-ml syringe. With the lamb in the prone position and tilted 45° to achieve right-side gravity dependence, half of the surfactant volume was instilled over ~15 s. This was done using a 5 French catheter cut to a length even with the distal opening of the endotracheal tube, through a NEO2-Safe adapter (B and B Medical Technologies, Carlsbad, CA) without disruption of positive end-expiratory pressure. The lamb was ventilated for 1 min. The lamb was then repositioned left side down and an identical dose of surfactant was administered, followed again by 1 min of ventilation. The lambs were not suctioned for at least 1 h after the administration of surfactant. The head of the bed was elevated approximately 15° to minimize regurgitation of the surfactant into the ventilator circuit.
Lyophilized lucinactant (Surfaxin LS, Discovery Laboratories, Warrington, PA) was administered as four divided bolus doses. The lyophilized product was reconstituted by adding 9.05 ml of water for injection (in accordance with United States Pharmacopoeia standards) at room temperature and shaken vigorously for at least 10 s to ensure the product was free-flowing. Lucinactant was drawn into a 10-ml syringe (based on 5.8 ml/kg and 175 mg/kg phospholipid) and administered in four quarter doses without interruption of the ventilatory circuit using a NEO2-Safe adapter. The lambs were positioned right side down for the first and third doses, and left side down for the second and fourth doses over 2 min of administration, as previously described in a clinical study with liquid instillate lucinactant (14). The lambs were not suctioned for 1 h after the administration of surfactant, and the head of the bed was elevated 15° to prevent significant regurgitation of the surfactant into the ventilator circuit.
Distribution
To assess the uniformity of surfactant distribution within the lungs, labeled microspheres were mixed to the surfactant administered to lambs in each study group. The microspheres (BioPAL, Worcester, MA) of 1–2 μm diameter (~30 million spheres with 7.2 mg of stable isotope metal) were provided in a water suspension. The spheres were centrifuged at 10,000g for 1 min, the supernatant was removed, and the pellet was suspended and mixed thoroughly with the surfactant solution immediately before administration as previously described (16).
At the end of the experiment, the lambs were killed, and the lungs were removed from the chest en bloc, placed in supine position on a flat surface, and frozen at −70 °C. The frozen lungs were weighed and then sectioned with three longitudinal slices cut along the sagittal plane, three cross-sectional slices cut along the transverse plane, and one longitudinal slice cut along the coronal plane (a total of 72 pieces) as previously described (15). Each piece was weighed immediately to minimize errors as a result of evaporative loss. The microspheres were quantified by neutron radioactivation of the metals followed by gamma counter analysis, resulting in a measurement of disintegrations per minute (dpm; BioPAL) in pieces of known weight and location.
Blood Pressure, Heart Rate, and Respiratory System Function
Arterial blood pressure was sampled at 200 Hz throughout the experiments using a pressure transducer (Cobe, Lakewood, CO), converted to a digital signal (Powerlab, ADInstruments, Colorado Springs, CO), and recorded by computer (Chart version 5.2 for Macintosh, ADInstruments). Heart rate was calculated from the arterial blood pressure waveform. Airway pressure and flow were monitored continuously through a variable orifice pneumotachometer (Bicore I, Cardinal Health, Dublin, OH). Pulmonary compliance (ml/cmH2O) and exhaled tidal volume (ml/kg) were recorded at 20 and 5 min before administration of surfactant and every 15 min thereafter.
Blood Gas Analyses
Arterial blood gas (0.4-ml blood sample), including PO2, PCO2, pH (ABL-5, Radiometer, Copenhagen, Denmark), and oxyhemoglobin saturation and O2 content (OSM3, Radiometer) were measured at −20, −15, −5, 5, 10, 20, and 30 min relative to the administration of surfactant, and then in 15-min intervals relative to the initiation of surfactant throughout the remainder of the experiment.
CBF and Oxygen Tension
CBF was sampled at 100 Hz via laser Doppler flowmetry (OxyFlo, Oxford Optronix, Oxford, UK). Because laser Doppler flowmetry measures relative changes in flow, values were expressed as a percent of mean baseline flow during the 20-min period immediately after the administration of surfactant. Cerebral brain tissue oxygen tensions was recorded at 10 Hz (OxyLite, Oxford Optronix). The OxyLite probe provides an absolute measure of tissue oxygenation by ruthenium dye fluorescence.
Data Analysis
To assess rapid changes after surfactant administration, the continuously recorded parameters (CBF, brain tissue oxygen tensions, blood pressure, and heart rate) were re-sampled into 10-s averages from 5 min before until 15 min after initiation of surfactant administration. The continuously recorded data were also re-sampled into 1-min averages from 20 min before until 180 min after initiation of administration of surfactant. The significant changes in parameters with time were assessed by one-way ANOVA with repeated measures (GraphPad Prism version 5.0 for Macintosh, GraphPad Software, La Jolla, CA). If the one-way ANOVA reached significance, a Bonferroni adjustment was used to determine which time points were measurably different from baseline. For blood gas, CRS, and tidal volume, measurements at −5 min were used as a baseline. Significant differences between the lyophilized lucinactant and poractant alfa groups were detected using two-way ANOVA.
Surfactant distribution in each tissue sample was normalized to tissue weight such that relative distribution was expressed as:

These results were expressed as logarithms (base 10) to achieve a normal distribution. In this manner, a perfectly homogenous microsphere distribution on a per-gram-tissue basis would have resulted in a value of 0 (log10 of 1) for all pieces. Data were evaluated to quantify differences in the microsphere distribution between the six upper-to-lower divisions, the three medial-to-lateral divisions, and the two ventral (dependent)-to-dorsal (nondependent) divisions. These assessments were made for both groups using one-way ANOVA for the upper-to-lower and medial-to-lateral divisions, and a t-test for the dependent-to-nondependent divisions. If a significant difference was found with ANOVA, Bonferroni’s and Dunnett’s adjustments were performed to identify differences between specific tissue sections. To demonstrate overall homogeneity between the two treatment groups, the distribution data are presented as a histogram as well as fit to a Gaussian curve (GraphPad Prism version 5.0 for Macintosh).
References
Clements J . Dependence of pressure-volume characteristics of lungs on intrinsic surface-active material. Am J Physiol 1956;187:592–6.
Avery ME, Mead J . Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 1959;97(5, Part 1):517–23.
Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T . Artificial surfactant therapy in hyaline-membrane disease. Lancet 1980;1:55–9.
Hallman M, Merritt TA, Schneider H, et al. Isolation of human surfactant from amniotic fluid and a pilot study of its efficacy in respiratory distress syndrome. Pediatrics 1983;71:473–82.
Enhorning G, Shennan A, Possmayer F, Dunn M, Chen CP, Milligan J . Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics 1985;76:145–53.
Kwong MS, Egan EA, Notter RH, Shapiro DL . Double-blind clinical trial of calf lung surfactant extract for the prevention of hyaline membrane disease in extremely premature infants. Pediatrics 1985;76:585–92.
Clark JC, Wert SE, Bachurski CJ, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA 1995;92:7794–8.
Mazela J, Merritt TA, Gadzinowski J, Sinha S . Evolution of pulmonary surfactants for the treatment of neonatal respiratory distress syndrome and paediatric lung diseases. Acta Paediatr 2006;95:1036–48.
Soll RF, Blanco F . Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Sys Rev 2001:CD000144.
Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K; North American Study Group. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol 2004;21:109–19.
Cochrane CG, Revak SD . Pulmonary surfactant protein B (SP-B): structure-function relationships. Science 1991;254:566–8.
Cochrane CG, Revak SD . Protein-phospholipid interactions in pulmonary surfactant. The Parker B. Francis Lectureship. Chest 1994;105:Suppl 3:57S–62S.
Moya F, Sinha S, Gadzinowski J, et al.; SELECT and STAR Study Investigators. One-year follow-up of very preterm infants who received lucinactant for prevention of respiratory distress syndrome: results from 2 multicenter randomized, controlled trials. Pediatrics 2007;119:e1361–70.
Moya FR, Gadzinowski J, Bancalari E, et al.; International Surfaxin Collaborative Study Group. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics 2005;115:1018–29.
Terry MH, Merritt TA, Harding B, et al. Pulmonary distribution of lucinactant and poractant alfa and their peridosing hemodynamic effects in a preterm lamb model of respiratory distress syndrome. Pediatr Res 2010;68:193–8.
Polglase GR, Hillman NH, Pillow JJ, et al. Ventilation-mediated injury after preterm delivery of Ureaplasma parvum colonized fetal lambs. Pediatr Res 2010;67:630–5.
Buckberg GD, Luck JC, Payne DB, Hoffman JI, Archie JP, Fixler DE . Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol 1971;31:598–604.
Vidyasagar D, Maeta H, Raju TN, et al. Bovine surfactant (surfactant TA) therapy in immature baboons with hyaline membrane disease. Pediatrics 1985;75:1132–42.
Cummings JJ, Holm BA, Hudak ML, Hudak BB, Ferguson WH, Egan EA . A controlled clinical comparison of four different surfactant preparations in surfactant-deficient preterm lambs. Am Rev Respir Dis 1992;145:999–1004.
Gilliard N, Richman PM, Merritt TA, Spragg RG . Effect of volume and dose on the pulmonary distribution of exogenous surfactant administered to normal rabbits or to rabbits with oleic acid lung injury. Am Rev Respir Dis 1990;141:743–7.
van der Bleek J, Plötz FB, van Overbeek FM, et al. Distribution of exogenous surfactant in rabbits with severe respiratory failure: the effect of volume. Pediatr Res 1993;34:154–8.
Alonso C, Alig T, Yoon J, Bringezu F, Warriner H, Zasadzinski JA . More than a monolayer: relating lung surfactant structure and mechanics to composition. Biophys J 2004;87:4188–202.
Espinosa FF, Shapiro AH, Fredberg JJ, Kamm RD . Spreading of exogenous surfactant in an airway. J Appl Physiol 1993;75:2028–39.
Espinosa FF, Kamm RD . Meniscus formation during tracheal instillation of surfactant. J Appl Physiol 1998;85:266–72.
Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG . Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: a comparison of laser Doppler and microsphere measurements of CBF. J Physiol (Lond) 2003;546(Pt 3):869–78.
Tsai AG, Johnson PC, Intaglietta M . Oxygen gradients in the microcirculation. Physiol Rev 2003;83:933–63.
Drysdale DB, Petersen ES . Arterial chemoreceptors, ventilation and heart rate in man. J Physiol (Lond) 1977;273:109–20.
Søvik S, Lossius K, Walløe L . Heart rate response to transient chemoreceptor stimulation in term infants is modified by exposure to maternal smoking. Pediatr Res 2001;49:558–65.
Acknowledgements
The authors are grateful for the skillful technical assistance of Shannon Bragg, Jeanette Merrill-Henry, and Jonathon Ross. Timothy Gregary is an employee of Discovery Laboratories, and Jan Mazela is a paid consultant of Discovery Laboratories.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazela, J., Merritt, T., Terry, M. et al. Comparison of poractant alfa and lyophilized lucinactant in a preterm lamb model of acute respiratory distress. Pediatr Res 72, 32–37 (2012). https://doi.org/10.1038/pr.2012.46
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.46
This article is cited by
-
Aerosol drug delivery to spontaneously-breathing preterm neonates: lessons learned
Respiratory Research (2021)
-
Mass spectrometry imaging as a tool for evaluating the pulmonary distribution of exogenous surfactant in premature lambs
Respiratory Research (2019)