Abstract
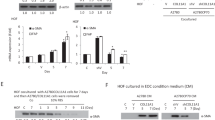
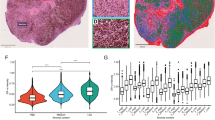
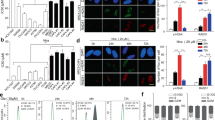
The signaling mediated by c-MET and its ligand, hepatocyte growth factor (HGF), has been implicated in malignant progression of cancer involving stimulation of proliferation, invasion and metastasis. We studied the c-MET/HGF axis as a mediator of tumor–stromal interaction in ovarian cancer and the value of targeting c-MET for the treatment of ovarian cancer. To assess c-MET signaling, we established in vitro models of the microenvironment using primary and immortalized human fibroblasts from normal ovary and tumor samples and epithelial ovarian cancer cell lines. We found that fibroblast from normal ovaries secreted high levels of HGF (1500–3800 pg/ml) as compared with tumor-derived fibroblasts (undetectable level) and could elicit cellular biological responses on c-MET-expressing ovarian cancer cells including increase of cell proliferation and migration (2- to 140-fold increase). HGF secreted by fibroblasts was also found sequestered within extracellular matrices (ECMs) and when degraded this ECM-derived HGF stimulated cancer cell migration (1.5- to 24-fold). In cells containing constitutive c-MET phosphorylation, recombinant HGF and fibroblast-derived HGF negligibly affect c-MET phosphorylation on Tyr1234 and Tyr1003. However, both sources of HGF increased the phosphorylation of c-MET on Tyr1349, the multi-substrate docking site, by more than sixfold and led to activation of downstream signaling transducers. DCC-2701 (Deciphera Pharmaceuticals, LLC), a novel c-MET/TIE-2/VEGFR inhibitor was able to effectively reduce tumor burden in vivo and block c-MET pTyr1349-mediated signaling, cell growth and migration as compared with a HGF antagonist in vitro. Importantly, DCC-2701’s anti-proliferative activity was dependent on c-MET activation induced by stromal human fibroblasts and to a lesser extent exogenous HGF. Our data suggest for the first time that DCC-2701 may be superior to HGF antagonists that are in clinical trials and that pTyr1349 levels might be a good indicator of c-MET activation and likely response to targeted therapy as a result of signals from the microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30.
Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ et al. Focus on epithelial ovarian cancer. Cancer Cell 2004; 5: 19–24.
Bhowmick NA, Moses HL . Tumor-stroma interactions. Curr Opin Genet Dev 2005; 15: 97–101.
Liotta LA, Kohn EC . The microenvironment of the tumour-host interface. Nature 2001; 411: 375–379.
Beacham DA, Cukierman E . Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol 2005; 15: 329–341.
Hynes RO . The extracellular matrix: not just pretty fibrils. Science 2009; 326: 1216–1219.
Matsumoto K, Nakamura T . Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer 2006; 119: 477–483.
Sierra JR, Tsao MS . c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol 2011; 3 (1 Suppl): S21–S35.
Corps AN, Sowter HM, Smith SK . Hepatocyte growth factor stimulates motility, chemotaxis and mitogenesis in ovarian carcinoma cells expressing high levels of c-met. Int J Cancer 1997; 73: 151–155.
Taipale J, Keski-Oja J . Growth factors in the extracellular matrix. FASEB J 1997; 11: 51–59.
Tatsumi R, Allen RE . Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve 2004; 30: 654–658.
Comoglio PM, Giordano S, Trusolino L . Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008; 7: 504–516.
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012; 487: 500–504.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012; 487: 505–509.
Ayhan A, Ertunc D, Tok EC, Ayhan A . Expression of the c-Met in advanced epithelial ovarian cancer and its prognostic significance. Int J Gynecol Cancer 2005; 15: 618–623.
Zillhardt M, Christensen JG, Lengyel E . An orally available small-molecule inhibitor of c-Met, PF-2341066, reduces tumor burden and metastasis in a preclinical model of ovarian cancer metastasis. Neoplasia 2010; 12: 1–10.
Zillhardt M, Park SM, Romero IL, Sawada K, Montag A, Krausz T et al. Foretinib (GSK1363089), an orally available multikinase inhibitor of c-Met and VEGFR-2, blocks proliferation, induces anoikis, and impairs ovarian cancer metastasis. Clin Cancer Res 2011; 17: 4042–4051.
Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res 2010; 16: 699–710.
Liu X, Newton RC, Scherle PA . Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med 2009; 16: 37–45.
Lyon M, Deakin JA, Mizuno K, Nakamura T, Gallagher JT . Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J Biol Chem 1994; 269: 11216–11223.
Ashikari S, Habuchi H, Kimata K . Characterization of heparan sulfate oligosaccharides that bind to hepatocyte growth factor. J Biol Chem 1995; 270: 29586–29593.
Zioncheck TF, Richardson L, Liu J, Chang L, King KL, Bennett GL et al. Sulfated oligosaccharides promote hepatocyte growth factor association and govern its mitogenic activity. J Biol Chem 1995; 270: 16871–16878.
Flynn DL, Ahn YM, Berger MS, Caldwell T, Hood MM, Kaufman MD et alRebastinib and DCC-2701: targeting of resistance mechanisms in cancer treatment. ACS National Meeting, New Orleans, 8 April, MEDI 209 2013.
Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny H, Becker AR et al. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res 2007; 67: 1670–1679.
Kwon Y, Cukierman E, Godwin AK . Differential expressions of adhesive molecules and proteases define mechanisms of ovarian tumor cell matrix penetration/invasion. PLoS One 2011; 6: e18872.
Furge KA, Zhang YW, Vande Woude GF . Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene 2000; 19: 5582–5589.
Pan BS, Chan GK, Chenard M, Chi A, Davis LJ, Deshmukh SV et al. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res 2010; 70: 1524–1533.
Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994; 77: 261–271.
Michieli P, Basilico C, Pennacchietti S, Maffe A, Tamagnone L, Giordano S et al. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene 1999; 18: 5221–5231.
Miyata Y, Kanetake H, Kanda S . Presence of phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res 2006; 12: 4876–4881.
Marchand-Adam S, Marchal J, Cohen M, Soler P, Gerard B, Castier Y et al. Defect of hepatocyte growth factor secretion by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003; 168: 1156–1161.
Matsuoka H, Sisson TH, Nishiuma T, Simon RH . Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am J Respir Cell Mol Biol 2006; 35: 705–713.
Lyon M, Deakin JA, Rahmoune H, Fernig DG, Nakamura T, Gallagher JT . Hepatocyte growth factor/scatter factor binds with high affinity to dermatan sulfate. J Biol Chem 1998; 273: 271–278.
Nayeri F, Nayeri T, Aili D, Brudin L, Liedberg B . Clinical impact of real-time evaluation of the biological activity and degradation of hepatocyte growth factor. Growth Factors 2008; 26: 163–171.
Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW et al. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res 1983; 43: 5379–5389.
Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res 1988; 48: 6166–6172.
Quiros RM, Valianou M, Kwon Y, Brown KM, Godwin AK, Cukierman E . Ovarian normal and tumor-associated fibroblasts retain in vivo stromal characteristics in a 3-D matrix-dependent manner. Gynecol Oncol 2008; 110: 99–109.
Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E . Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol 2005; 167: 475–488.
Beacham DA, Amatangelo MD, Cukierman E . Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol 2007, Chapter 10 Unit 10 9.
Castello-Cros R, Cukierman E . Stromagenesis during tumorigenesis: characterization of tumor-associated fibroblasts and stroma-derived 3D matrices. Methods Mol Biol 2009; 522: 275–305.
Cukierman E, Pankov R, Stevens DR, Yamada KM . Taking cell-matrix adhesions to the third dimension. Science 2001; 294: 1708–1712.
Acknowledgements
We thank Dr Ziyan Pessetto for her expertise in the cell viability assays and Dr Daniel Flynn for his helpful comments and guidance in regards the biology and activity of DCC-2701. The study was supported in part by a program project grant from Ovarian Cancer Research Fund (http://www.ocrf.org) and a grant from the NCI (R01 CA140323) to AKG. We would also like to acknowledge support from the University of Kansas Cancer Center’s CCSG (P30 CA168524), the Kansas Bioscience Authority Eminent Scholar Program. AKG is the Chancellors Distinguished Chair in Biomedical Sciences endowed Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr AKG’s work has been funded by the NIH/NCI, the state of Kansas and non-profit organizations. BSD and MDK are employees of and receive compensation from Deciphera Pharmaceuticals, LLC. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kwon, Y., Smith, B., Zhou, Y. et al. Effective inhibition of c-MET-mediated signaling, growth and migration of ovarian cancer cells is influenced by the ovarian tissue microenvironment. Oncogene 34, 144–153 (2015). https://doi.org/10.1038/onc.2013.539
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.539
Keywords
This article is cited by
-
Loss of LECT2 promotes ovarian cancer progression by inducing cancer invasiveness and facilitating an immunosuppressive environment
Oncogene (2024)
-
Deciphering tumor immune microenvironment differences between high-grade serous and endometrioid ovarian cancer to investigate their potential in indicating immunotherapy response
Journal of Ovarian Research (2023)
-
Ascitic fluid shear stress in concert with hepatocyte growth factor drive stemness and chemoresistance of ovarian cancer cells via the c-Met-PI3K/Akt-miR-199a-3p signaling pathway
Cell Death & Disease (2022)
-
Epithelial-stromal communication via CXCL1-CXCR2 interaction stimulates growth of ovarian cancer cells through p38 activation
Cellular Oncology (2021)
-
Autocrine HGF/c-Met signaling pathway confers aggressiveness in lymph node adult T-cell leukemia/lymphoma
Oncogene (2020)