Abstract
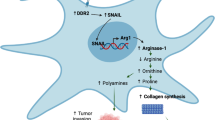
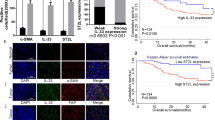
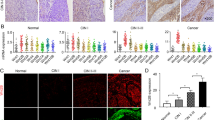
Ovarian cancer has a unique tumor microenvironment (TME) that enables cancer-associated fibroblasts (CAFs) to interact with cellular and matrix constituents and influence tumor development and migration into the peritoneal cavity. Collagen type XI alpha 1 (COL11A1) is overexpressed in CAFs; therefore this study examines its role during CAF activation in epithelial ovarian cancer (EOC). Coculturing human ovarian fibroblasts (HOFs) with high COL11A1-expressing EOC cells or exposure to the conditioned medium of these cells prompted the expression of COL11A1 and CAF phenotypes. Conversely, coculturing HOFs with low COL11A1-expressing EOC cells or COL11A1-knockdown abrogated COL11A1 overexpression and secretion, in addition to CAF activation. Increased p-SP1 expression attributed to COL11A1-mediated extracellular signal-regulated kinase activation (ERK) induced p65 translocation into the nucleus and augmented its binding to the insulin-like growth factor binding protein 2 (IGFBP2) promoter, ultimately inducing TGF-β3 activation. The CAF–cancer cell crosstalk triggered interleukin-6 release, which in turn promoted EOC cell proliferation and invasiveness. These in vitro results were confirmed by in vivo findings in a mouse model, showing that COL11A1 overexpression in EOC cells promoted tumor formation and CAF activation, which was inhibited by TGF-β3 antibody. Human tumors with high TGF-β3 levels showed elevated expression of COL11A1 and IGFBP2, which was associated with poor survival. Our findings suggest the possibility that anti-TGF-β3 treatment strategy may be effective in targeting CAFs in COL11A1-positive ovarian tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020. https://doi.org/10.1038/s41568-019-0238-1.
D’Arcangelo E, Wu NC, Cadavid JL, McGuigan AP. The life cycle of cancer-associated fibroblasts within the tumour stroma and its importance in disease outcome. Br J Cancer. 2020. https://doi.org/10.1038/s41416-019-0705-1.
Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Cancer Genome Atlas Research Network, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256.
Worzfeld T, Pogge von Strandmann E, Huber M, Adhikary T, Wagner U, Reinartz S, et al. The unique molecular and cellular microenvironment of ovarian cancer. Front Oncol. 2017;7:24.
Pogge von Strandmann E, Reinartz S, Wager U, Müller R. Tumor-host cell interactions in ovarian cancer: pathways to therapy failure. Trends Cancer. 2017;3:137–48.
Wu YH, Chang TH, Huang YF, Huang HD, Chou CY. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene. 2014;33:3432–40.
Wu YH, Chang TH, Huang YF, Chen CC, Chou CY. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPβ pathway and PDK1 stabilization. Oncotarget. 2015;6:23748–63.
Wu YH, Huang YF, Chang TH, Chou CY. Activation of TWIST1 by COL11A1 promotes chemoresistance and inhibits apoptosis in ovarian cancer cells by modulating NF-κB-mediated IKKβ expression. Int J Cancer. 2017;141:2305–17.
Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, et al. A collagen-remodeling gene signature regulated by TGF-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res. 2014;20:711–23.
Rada M, Nallanthighal S, Cha J, Ryan K, Sage J, Eldred C, et al. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI Alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene. 2018;37:4809–20.
Nallanthighal S, Rada M, Heiserman JP, Cha J, Sage J, Zhou B, et al. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell Death Dis. 2020;11:258.
Fischer H, Stenling R, Rubio C, Lindblom A. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis. 2001;22:875–8.
Navab R, Strumpf D, Bandarchi B, Zhu CQ, Pintilie M, Ramnarine VR, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci USA. 2011;108:7160–5.
Erkan M, Weis N, Pan Z, Schwager C, Samkharadze T, Jiang X, et al. Organ-, inflammation- and cancer specific transcriptional fingerprints of pancreatic and hepatic stellate cells. Mol Cancer. 2010;9:88.
García-Pravia C, Galván JA, Gutiérrez-Corral N, Solar-García L, García-Pérez E, García-Ocaña M, et al. Overexpression of COL11A1 by cancer-associated fibroblasts: clinical relevance of a stromal marker in pancreatic cancer. PLoS ONE. 2013;8:e78327.
Park H, Lee Y, Lee H, Kim JW, Hwang JH, Kim J, et al. The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumour Biol. 2017;39:1010428317718403.
Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, et al. TGF-β modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013;73:5016–28.
Cazals V, Nabeyrat E, Corroyer S, de Keyzer Y, Clement A. Role for NF-kappa B in mediating the effects of hyperoxia on IGF-binding protein 2 promoter activity in lung alveolar epithelial cells. Biochim Biophys Acta. 1999;1448:349–62.
Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. 2010;15:166–79.
Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611–20.
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020. https://doi.org/10.1038/s41568-019-0238-1.
Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, et al. IL1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9:282–301.
Mienaltowski MJ, Birk DE. Structure, physiology, and biochemistry of collagens. Adv Exp Med Biol. 2014;802:5–29.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–79.e10.
Stover DG, Bierie B, Moses HL. A delicate balance: TGF-β and the tumor microenvironment. J Cell Biochem. 2007;101:851–61. Review.
Verona EV, Elkahloun AG, Yang J, Bandyopadhyay A, Yeh IT, Sun LZ. Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 2007;67:5737–46.
Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–99.
Papageorgis P. TGFβ Signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193.
Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, et al. A collagen-remodeling gene signature regulated by TGF-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res. 2014;20:711–23.
Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, et al. Transforming growth factor-β regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–19.
Barcellos-Hoff MH, Medina D. New highlights on stroma-epithelial interactions in breast cancer. Breast Cancer Res. 2004;7:33–6.
Zigrino P, Löffek S, Mauch C. Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie. 2005;87:321–8.
Hawsawi NM, Ghebeh H, Hendrayani SF, Tulbah A, Al-Eid M, Al-Tweigeri T, et al. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008;68:2717–25.
Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Investig. 1995;95:859–73.
Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–88. Review.
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–32.
Bartlett JM, Langdon SP, Scott WN, Love SB, Miller EP, Katsaros D, et al. Transforming growth factor-beta isoform expression in human ovarian tumours. Eur J Cancer. 1997;33:2397–403.
Hong SH, Ondrey FG, Avis IM, Chen Z, Loukinova E, Cavanaugh PF Jr., et al. Cyclooxygenase regulates human oropharyngeal carcinomas via the proinflammatory cytokine IL-6: a general role for inflammation? FASEB J. 2000;14:1499–507.
Bran G, Gotte K, Riedel K, Hormann K, Riedel F. IL-6 antisense-mediated growth inhibition in a head and neck squamous cell carcinoma cell line. In Vivo. 2011;25:579–84.
Chen MF, Wang WH, Lin PY, Lee KD, Chen WC. Significance of the TGF-beta1/IL-6 axis in oral cancer. Clin Sci. 2012;122:459–72.
Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–97.
Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res. 2018;10:6685–93.
Acknowledgements
This work was supported by grants from the National Science Council (MOST: No. 108-2314-B-384-011-MY3 and 108-2314-B-006-061-MY2). The study was also supported by grants from the Chi Mei Medical Center, Liouying Campus (CLFHR10822, CLFHR10911, CMLMOST10901, and CLFHR10921).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, YH., Huang, YF., Chang, TH. et al. COL11A1 activates cancer-associated fibroblasts by modulating TGF-β3 through the NF-κB/IGFBP2 axis in ovarian cancer cells. Oncogene 40, 4503–4519 (2021). https://doi.org/10.1038/s41388-021-01865-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01865-8
This article is cited by
-
COL5A1 promotes triple-negative breast cancer progression by activating tumor cell-macrophage crosstalk
Oncogene (2024)
-
Single-cell landscape of undifferentiated pleomorphic sarcoma
Oncogene (2024)
-
Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments
Molecular Cancer (2023)
-
The downregulation of miR-509-3p expression by collagen type XI alpha 1-regulated hypermethylation facilitates cancer progression and chemoresistance via the DNA methyltransferase 1/Small ubiquitin-like modifier-3 axis in ovarian cancer cells
Journal of Ovarian Research (2023)
-
Extracellular matrix educates an immunoregulatory tumor macrophage phenotype found in ovarian cancer metastasis
Nature Communications (2023)