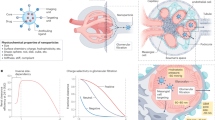
Key Points
-
Oncology has benefited greatly from nanotechnology research and investment, yielding important insights that are applicable to kidney nanomedicines
-
Nanocarriers have the potential to improve the pharmacokinetics, biodistribution, toxicity, and efficacy of drugs
-
Delivery and retention of nanomedicines in the kidney is challenging and requires a deep understanding of the renal barriers and effective engineering of nanoparticle size, shape, and surface chemistry
-
To improve drug efficacy and minimize systemic toxicity, nanomedicines can be targeted to distinct kidney cell types and/or to extracellular matrix components such as the glomerular basement membrane
-
Nanocarriers can deliver drugs, proteins, peptides and nucleic acids, and can facilitate the targeted delivery of genes or RNA interference molecules to treat kidney disorders for which the efficacy of current treatments is limited
-
Organ-on-a-chip kidneys can streamline the simultaneous screening and testing of nanomedicines in environments that mimic the kidney and accelerate translation of kidney-specific therapeutics
Abstract
Treatment and management of kidney disease currently presents an enormous global burden, and the application of nanotechnology principles to renal disease therapy, although still at an early stage, has profound transformative potential. The increasing translation of nanomedicines to the clinic, alongside research efforts in tissue regeneration and organ-on-a-chip investigations, are likely to provide novel solutions to treat kidney diseases. Our understanding of renal anatomy and of how the biological and physico-chemical properties of nanomedicines (the combination of a nanocarrier and a drug) influence their interactions with renal tissues has improved dramatically. Tailoring of nanomedicines in terms of kidney retention and binding to key membranes and cell populations associated with renal diseases is now possible and greatly enhances their localization, tolerability, and efficacy. This Review outlines nanomedicine characteristics central to improved targeting of renal cells and highlights the prospects, challenges, and opportunities of nanotechnology-mediated therapies for renal diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Farokhzad, O. C. & Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20 (2009).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Wang, A. Z., Langer, R. & Farokhzad, O. C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 63, 185–198 (2012).
Heath, J. R. & Davis, M. E. Nanotechnology and cancer. Annu. Rev. Med. 59, 251–265 (2008).
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 7, 771–782 (2008).
Wang, J., Byrne, J. D., Napier, M. E. & DeSimone, J. M. More effective nanomedicines through particle design. Small 7, 1919–1931 (2011).
Saha, K., Bajaj, A., Duncan, B. & Rotello, V. M. Beauty is skin deep: a surface monolayer perspective on nanoparticle interactions with cells and bio-macromolecules. Small 7, 1903–1918 (2011).
Mu, Q. et al. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 114, 7740–7781 (2014).
Albanese, A. et al. Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano 8, 5515–5526 (2014).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009).
Gratton, S. E. et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA 105, 11613–11618 (2008).
Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F. & Farokhzad, O. C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41, 2971–3010 (2012).
Anselmo, A. C. & Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. http://dx.doi.org/10.1016/j.addr.2016.01.007 (2016).
Marty, J. J., Oppenheim, R. C. & Speiser, P. Nanoparticles — a new colloidal drug delivery system. Pharm. Acta Helv. 53, 17–23 (1978).
Shi, J., Xiao, Z., Kamaly, N. & Farokhzad, O. C. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc. Chem. Res. 44, 1123–1134 (2011).
Chen, G., Roy, I., Yang, C. & Prasad, P. N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 116, 2826–2885 (2016).
Stuart, M. A. et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 9, 101–113 (2010).
Pacardo, D. B., Ligler, F. S. & Gu, Z. Programmable nanomedicine: synergistic and sequential drug delivery systems. Nanoscale 7, 3381–3391 (2015).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Koetting, M. C., Peters, J. T., Steichen, S. D. & Peppas, N. A. Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater. Sci. Eng. R. Rep. 93, 1–49 (2015).
Torchilin, V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 13, 813–827 (2014).
de la Rica, R., Aili, D. & Stevens, M. M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 64, 967–978 (2012).
Correa, S., Dreaden, E. C., Gu, L. & Hammond, P. T. Engineering nanolayered particles for modular drug delivery. J. Control. Release 240, 364–386 (2016).
Kemp, J. A., Shim, M. S., Heo, C. Y. & Kwon, Y. J. “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 98, 3–18 (2016).
Xu, X., Ho, W., Zhang, X., Bertrand, N. & Farokhzad, O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol. Med. 21, 223–232 (2015).
Tabernero, J. et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 3, 406–417 (2013).
Reddy, L. H. & Couvreur, P. Nanotechnology for therapy and imaging of liver diseases. J. Hepatol. 55, 1461–1466 (2011).
Wang, A. Z. et al. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 8, 1063–1070 (2008).
Lyseng-Williamson, K. A., Duggan, S. T. & Keating, G. M. Pegylated liposomal doxorubicin: a guide to its use in various malignancies. BioDrugs 27, 533–540 (2013).
Barenholz, Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012).
Harrison, M., Tomlinson, D. & Stewart, S. Liposomal-entrapped doxorubicin: an active agent in AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 13, 914–920 (1995).
Money-Kyrle, J. F. et al. Liposomal daunorubicin in advanced Kaposi's sarcoma: a phase II study. Clin. Oncol. 5, 367–371 (1993).
Rosenthal, E. et al. Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcoma. Am. J. Clin. Oncol. 25, 57–59 (2002).
Meyerhoff, A. U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin. Infect. Dis. 28, 42–48 (1999).
Khemapech, N., Oranratanaphan, S., Termrungruanglert, W., Lertkhachonsuk, R. & Vasurattana, A. Salvage chemotherapy in recurrent platinum-resistant or refractory epithelial ovarian cancer with Carboplatin and distearoylphosphatidylcholine pegylated liposomal Doxorubicin (Lipo-Dox®). Asian Pac. J. Cancer Prev. 14, 2131–2135 (2013).
Glantz, M. J. et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 5, 3394–3402 (1999).
Batist, G. et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 19, 1444–1454 (2001).
Agosta, E. et al. Pharmacogenetics of antiangiogenic and antineovascular therapies of age-related macular degeneration. Pharmacogenomics 13, 1037–1053 (2012).
Gabizon, A. et al. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother. Pharmacol. 61, 695–702 (2008).
Gambling, D., Hughes, T., Martin, G., Horton, W. & Manvelian, G. A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth. Analg. 100, 1065–1074 (2005).
Venkatakrishnan, K. et al. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br. J. Clin. Pharmacol. 77, 998–1010 (2014).
Ingram, I. FDA approves liposomal vincristine (Marqibo) for rare leukemia. Oncology (Williston Park) 26, 841 (2012).
Silverman, J. A. & Deitcher, S. R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 71, 555–564 (2013).
Allen, T. M. & Cullis, P. R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48 (2013).
Gabizon, A., Shmeeda, H. & Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 42, 419–436 (2003).
Kratz, F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 132, 171–183 (2008).
Singla, A. K., Garg, A. & Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 235, 179–192 (2002).
Kundranda, M. N. & Niu, J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des. Devel. Ther. 9, 3767–3777 (2015).
Liu, Z. & Chen, X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 45, 1432–1456 (2016).
Ibrahim, N. K. et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J. Clin. Oncol. 23, 6019–6026 (2005).
Rajeshkumar, N. V. et al. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br. J. Cancer 115, 442–453 (2016).
Park, S. R. et al. A multi-center, late phase II clinical trial of Genexol (paclitaxel) and cisplatin for patients with advanced gastric cancer. Oncol. Rep. 12, 1059–1064 (2004).
Kim, T. Y. et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 10, 3708–3716 (2004).
Ediriwickrema, A., Zhou, J., Deng, Y. & Saltzman, W. M. Multi-layered nanoparticles for combination gene and drug delivery to tumors. Biomaterials 35, 9343–9354 (2014).
Talelli, M. et al. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today 10, 93–117 (2015).
Nishiyama, N., Matsumura, Y. & Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 107, 867–874 (2016).
Cabral, H. & Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 190, 465–476 (2014).
Batrakova, E. V. & Kabanov, A. V. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 130, 98–106 (2008).
Maeda, H., Bharate, G. Y. & Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm 71, 409–419 (2009).
Oldham, E. A. Li, C., Ke, S., Wallace, S. & Huang, P. Comparison of action of paclitaxel and poly(L-glutamic acid)-paclitaxel conjugate in human breast cancer cells. Int. J. Oncol. 16, 125–132 (2000).
Duncan, R. Polymer therapeutics: top 10 selling pharmaceuticals — what next? J. Control. Release 190, 371–380 (2014).
Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 6, 688–701 (2006).
Espelin, C. W., Leonard, S. C., Geretti, E., Wickham, T. J. & Hendriks, B. S. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 76, 1517–1527 (2016).
Hrkach, J. et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl Med. 4, 128ra139 (2012).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010).
Lancet, J. E. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J. Clin. Oncol. 34 (Suppl.), 7000 (2016).
Kannan, R. M., Nance, E., Kannan, S. & Tomalia, D. A. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J. Intern. Med. 276, 579–617 (2014).
Roy, U. et al. The potential of HIV-1 nanotherapeutics: from in vitro studies to clinical trials. Nanomedicine (Lond.) 10, 3597–3609 (2015).
Mignani, S., El Kazzouli, S., Bousmina, M. & Majoral, J. P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv. Drug Deliv. Rev. 65, 1316–1330 (2013).
Dreaden, E. C., Mackey, M. A., Huang, X., Kang, B. & El-Sayed, M. A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 40, 3391–3404 (2011).
Anselmo, A. C. & Mitragotri, S. A. Review of clinical translation of inorganic nanoparticles. AAPS J. 17, 1041–1054 (2015).
Giljohann, D. A. et al. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 49, 3280–3294 (2010).
Paithankar, D. et al. Ultrasonic delivery of silica-gold nanoshells for photothermolysis of sebaceous glands in humans: Nanotechnology from the bench to clinic. J. Control Release 206, 30–36 (2015).
Yang, Y. & Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine 12, 317–332 (2016).
Meng, H. et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 9, 3540–3557 (2015).
Laurent, S. et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008).
Maier-Hauff, K. et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron–oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 103, 317–324 (2011).
Maggiorella, L. et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 8, 1167–1181 (2012).
Field, J. A. et al. Cytotoxicity and physicochemical properties of hafnium oxide nanoparticles. Chemosphere 84, 1401–1407 (2011).
Libutti, S. K. et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 16, 6139–6149 (2010).
Li, J., Gupta, S. & Li, C. Research perspectives: gold nanoparticles in cancer theranostics. Quant. Imaging Med. Surg. 3, 284–291 (2013).
Fortuin, A. S., Meijer, H., Thompson, L. C., Witjes, J. A. & Barentsz, J. O. Ferumoxtran-10 ultrasmall superparamagnetic iron oxide-enhanced diffusion-weighted imaging magnetic resonance imaging for detection of metastases in normal-sized lymph nodes in patients with bladder and prostate cancer: do we enter the era after extended pelvic lymph node dissection? Eur. Urol. 64, 961–963 (2013).
Hedgire, S. S. et al. Enhanced primary tumor delineation in pancreatic adenocarcinoma using ultrasmall super paramagnetic iron oxide nanoparticle-ferumoxytol: an initial experience with histopathologic correlation. Int. J. Nanomedicine 9, 1891–1896 (2014).
Rivera Gil, P., Huhn, D., del Mercato, L. L., Sasse, D. & Parak, W. J. Nanopharmacy: inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 62, 115–125 (2010).
Tolcher, A. W. et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 73, 363–371 (2014).
Schultheis, B. et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 32, 4141–4148 (2014).
Jensen, S. A. et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl Med. 5, 209ra152 (2013).
Islam, M. A. et al. Biomaterials for mRNA delivery. Biomater. Sci. 3, 1519–1533 (2015).
Park, J. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 11, 895–905 (2012).
Lee, I. H. et al. Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates. J. Control. Release 155, 435–441 (2011).
Park, B. H. et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 9, 533–542 (2008).
Yildiz, I., Shukla, S. & Steinmetz, N. F. Applications of viral nanoparticles in medicine. Curr. Opin. Biotechnol. 22, 901–908 (2011).
Czapar, A. E. et al. Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano 10, 4119–4126 (2016).
Parato, K. A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 20, 749–758 (2012).
Batrakova, E. V. & Kim, M. S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 219, 396–405 (2015).
Morrison, E. E., Bailey, M. A. & Dear, J. W. Renal extracellular vesicles: from physiology to clinical application. J. Physiol. 594, 5735–5748 (2016).
Alvarez, M. L., Khosroheidari, M., Kanchi Ravi, R. & DiStefano, J. K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 82, 1024–1032 (2012).
Bitzer, M., Ben-Dov, I. Z. & Thum, T. Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int. 82, 375–377 (2012).
Chow, E. K. et al. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci. Transl Med. 3, 73ra21 (2011).
Mochalin, V. N. et al. Adsorption of drugs on nanodiamond: toward development of a drug delivery platform. Mol. Pharm. 10, 3728–3735 (2013).
Ho, D. Nanodiamond-based chemotherapy and imaging. Cancer Treat. Res. 166, 85–102 (2015).
Jiang, T. et al. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv. Mater. 27, 1021–1028 (2015).
Liu, Z., Robinson, J. T., Sun, X. & Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 130, 10876–10877 (2008).
Valencia, P. M. et al. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine (Lond.) 8, 687–698 (2013).
Kim, J., Kim, J., Jeong, C. & Kim, W. J. Synergistic nanomedicine by combined gene and photothermal therapy. Adv. Drug Deliv. Rev. 98, 99–112 (2016).
Mura, S. & Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 64, 1394–1416 (2012).
Kenny, G. D. et al. Novel multifunctional nanoparticle mediates siRNA tumour delivery, visualisation and therapeutic tumour reduction in vivo. J. Control. Release 149, 111–116 (2011).
Liang, C., Xu, L., Song, G. & Liu, Z. Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem. Soc. Rev. http://dx.doi.org/10.1039/C6CS00458J (2016).
Hessel, C. M. et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 11, 2560–2566 (2011).
Ferreira, M. M., Bismuth, J. & Torresani, J. Reversible dissociation of triiodothyronine-nuclear receptor complexes by mercurial and chaotropic reagents. Biochem. Biophys. Res. Commun. 105, 244–251 (1982).
Maldonado, R. A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015).
Ilyinskii, P. O. et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 32, 2882–2895 (2014).
Swartz, M. A., Hirosue, S. & Hubbell, J. A. Engineering approaches to immunotherapy. Sci. Transl Med. 4, 148rv149 (2012).
Smith, D. M., Simon, J. K. & Baker, J. R. Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 13, 592–605 (2013).
Irvine, D. J., Swartz, M. A. & Szeto, G. L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 12, 978–990 (2013).
Rosenthal, J. A., Chen, L., Baker, J. L., Putnam, D. & DeLisa, M. P. Pathogen-like particles: biomimetic vaccine carriers engineered at the nanoscale. Curr. Opin. Biotechnol. 28, 51–58 (2014).
Stary, G. et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T. cells. Science 348, aaa8205 (2015).
Kodaira, H. et al. The targeting of anionized polyvinylpyrrolidone to the renal system. Biomaterials 25, 4309–4315 (2004).
Borgman, M. P. et al. Tumor-targeted HPMA copolymer-(RGDfK)–(CHX-A''-DTPA) conjugates show increased kidney accumulation. J. Control. Release 132, 193–199 (2008).
Qiao, H. et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials 35, 7157–7171 (2014).
Yuan, Z. X. et al. Specific renal uptake of randomly 50% N-acetylated low molecular weight chitosan. Mol. Pharm. 6, 305–314 (2009).
Dolman, M. E., Harmsen, S., Storm, G., Hennink, W. E. & Kok, R. J. Drug targeting to the kidney: advances in the active targeting of therapeutics to proximal tubular cells. Adv. Drug Deliv. Rev. 62, 1344–1357 (2010).
Leeuwis, J. W., Nguyen, T. Q., Dendooven, A., Kok, R. J. & Goldschmeding, R. Targeting podocyte-associated diseases. Adv. Drug Deliv. Rev. 62, 1325–1336 (2010).
Dolman, M. E. et al. Dendrimer-based macromolecular conjugate for the kidney-directed delivery of a multitargeted sunitinib analogue. Macromol. Biosci. 12, 93–103 (2012).
Yuan, Z. X. et al. Enhanced accumulation of low-molecular-weight chitosan in kidneys: a study on the influence of N-acetylation of chitosan on the renal targeting. J. Drug Target. 19, 540–551 (2011).
Gao, S. et al. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics 4, 1039–1051 (2014).
Suana, A. J. et al. Single application of low-dose mycophenolate mofetil-OX7-immunoliposomes ameliorates experimental mesangial proliferative glomerulonephritis. J. Pharmacol. Exp. Ther. 337, 411–422 (2011).
Tuffin, G., Huwyler, J., Waelti, E., Hammer, C. & Marti, H. P. Drug targeting using OX7-immunoliposomes: correlation between Thy1.1 antigen expression and tissue distribution in the rat. J. Drug Target. 16, 156–166 (2008).
Morimoto, K. et al. Advances in targeting drug delivery to glomerular mesangial cells by long circulating cationic liposomes for the treatment of glomerulonephritis. Pharm. Res. 24, 946–954 (2007).
Tuffin, G., Waelti, E., Huwyler, J., Hammer, C. & Marti, H. P. Immunoliposome targeting to mesangial cells: a promising strategy for specific drug delivery to the kidney. J. Am. Soc. Nephrol. 16, 3295–3305 (2005).
Scindia, Y., Deshmukh, U., Thimmalapura, P. R. & Bagavant, H. Anti-α8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: a novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis Rheum. 58, 3884–3891 (2008).
Monti, D. M. et al. Biocompatibility, uptake and endocytosis pathways of polystyrene nanoparticles in primary human renal epithelial cells. J. Biotechnol. 193, 3–10 (2015).
Chen, H. et al. Gd-encapsulated carbonaceous dots with efficient renal clearance for magnetic resonance imaging. Adv. Mater. 26, 6761–6766 (2014).
Zhang, X. D. et al. Passing through the renal clearance barrier: toward ultrasmall sizes with stable ligands for potential clinical applications. Int. J. Nanomedicine 9, 2069–2072 (2014).
Longmire, M., Choyke, P. L. & Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond.) 3, 703–717 (2008).
Zarschler, K. et al. Ultrasmall inorganic nanoparticles: state-of-the-art and perspectives for biomedical applications. Nanomedicine 12, 1663–1701 (2016).
Nair, A. V., Keliher, E. J., Core, A. B., Brown, D. & Weissleder, R. Characterizing the interactions of organic nanoparticles with renal epithelial cells in vivo. ACS Nano 9, 3641–3653 (2015).
Chen, X. et al. Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway. Int. J. Nanomedicine 10, 1–22 (2015).
L'Azou, B. et al. In vitro effects of nanoparticles on renal cells. Part. Fibre Toxicol. 5, 22 (2008).
Chen, Z. et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 163, 109–120 (2006).
Liao, J. et al. Effect of steroid-liposome on immunohistopathology of IgA nephropathy in ddY mice. Nephron 89, 194–200 (2001).
Ito, K. et al. Liposome-mediated transfer of nitric oxide synthase gene improves renal function in ureteral obstruction in rats. Kidney Int. 66, 1365–1375 (2004).
Pridgen, E. M., Alexis, F. & Farokhzad, O. C. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin. Drug Deliv. 12, 1459–1473 (2015).
Pridgen, E. M. et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci. Transl Med. 5, 213ra167 (2013).
Beloqui, A., des Rieux, A. & Preat, V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. http://dx.doi.org/10.1016/j.addr.2016.04.014 (2016).
Mitragotri, S., Burke, P. A. & Langer, R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 13, 655–672 (2014).
Zhu, X. et al. Polymeric nanoparticles amenable to simultaneous installation of exterior targeting and interior therapeutic proteins. Angew. Chem. Int. Ed. 55, 3309–3312 (2016).
Choi, K. Y., Liu, G., Lee, S. & Chen, X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale 4, 330–342 (2012).
Cheng, Z., Al Zaki, A., Hui, J. Z., Muzykantov, V. R. & Tsourkas, A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 338, 903–910 (2012).
Cedervall, T. et al. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. Int. Ed. 46, 5754–5756 (2007).
Lynch, I., Salvati, A. & Dawson, K. A. Protein–nanoparticle interactions: what does the cell see? Nat. Nanotechnol. 4, 546–547 (2009).
Cedervall, T. et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl Acad. Sci. USA 104, 2050–2055 (2007).
Lundqvist, M. et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105, 14265–14270 (2008).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Ritz, S. et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules 16, 1311–1321 (2015).
Ogawara, K. et al. Pre-coating with serum albumin reduces receptor-mediated hepatic disposition of polystyrene nanosphere: implications for rational design of nanoparticles. J. Control. Release 100, 451–455 (2004).
Mahmoudi, M. et al. Protein–nanoparticle interactions: opportunities and challenges. Chem. Rev. 111, 5610–5637 (2011).
Monopoli, M. P., Aberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012).
Chanan-Khan, A. et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann. Oncol. 14, 1430–1437 (2003).
Suk, J. S., Xu, Q., Kim, N., Hanes, J. & Ensign, L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99, 28–51 (2016).
Schottler, S. et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 11, 372–377 (2016).
Salvati, A. et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 8, 137–143 (2013).
Dong, Y. et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA 111, 3955–3960 (2014).
Sakulkhu, U. et al. Ex situ evaluation of the composition of protein corona of intravenously injected superparamagnetic nanoparticles in rats. Nanoscale 6, 11439–11450 (2014).
Walkey, C. D. et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455 (2014).
Bigdeli, A. et al. Exploring cellular interactions of liposomes using protein corona fingerprints and physicochemical properties. ACS Nano 10, 3723–3737 (2016).
Choi, C. H., Zuckerman, J. E., Webster, P. & Davis, M. E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl Acad. Sci. USA 108, 6656–6661 (2011).
Yang, Q. & Lai, S. K. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 7, 655–677 (2015).
Ishida, T. et al. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J. Control. Release 105, 305–317 (2005).
Ichihara, M. et al. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics 3, 1–11 (2010).
Dams, E. T. et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 292, 1071–1079 (2000).
Kalra, A. V. et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 74, 7003–7013 (2014).
Koizumi, F. et al. Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res. 66, 10048–10056 (2006).
Nakajima, T. E. et al. Antitumor effect of SN-38-releasing polymeric micelles, NK012, on spontaneous peritoneal metastases from orthotopic gastric cancer in mice compared with irinotecan. Cancer Res. 68, 9318–9322 (2008).
Hamaguchi, T. et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 92, 1240–1246 (2005).
Nagai, N. et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother. Pharmacol. 39, 131–137 (1996).
Barpe, D. R., Rosa, D. D. & Froehlich, P. E. Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur. J. Pharm. Sci. 41, 458–463 (2010).
Puelles, V. G. et al. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 7–15 (2011).
Eaton, D. C., Pooler, J. Vander's Renal Physiology 8th edn (McGraw-Hill Medical Publishing, 2013).
Satchell, S. The role of the glomerular endothelium in albumin handling. Nat. Rev. Nephrol. 9, 717–725 (2013).
Miner, J. H. The glomerular basement membrane. Exp. Cell Res. 318, 973–978 (2012).
Armelloni, S. et al. Podocytes: recent biomolecular developments. Biomol. Concepts 5, 319–330 (2014).
Abboud, H. E. Mesangial cell biology. Exp. Cell Res. 318, 979–985 (2012).
Shankland, S. J., Smeets, B., Pippin, J. W. & Moeller, M. J. The emergence of the glomerular parietal epithelial cell. Nat. Rev. Nephrol. 10, 158–173 (2014).
Brown, D. & Wagner, C. A. Molecular mechanisms of acid–base sensing by the kidney. J. Am. Soc. Nephrol. 23, 774–780 (2012).
Chang, R. L., Deen, W. M., Robertson, C. R. & Brenner, B. M. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 8, 212–218 (1975).
Mallipattu, S. K. & He, J. C. A new mechanism for albuminuria-induced podocyte injury. J. Am. Soc. Nephrol. 24, 1709–1711 (2013).
Choi, H. S. et al. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 5, 42–47 (2010).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Zuckerman, J. E. & Davis, M. E. Targeting therapeutics to the glomerulus with nanoparticles. Adv. Chronic Kidney Dis. 20, 500–507 (2013).
Toy, R., Peiris, P. M., Ghaghada, K. B. & Karathanasis, E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine (Lond.) 9, 121–134 (2014).
Rolland, J. P. et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 127, 10096–10100 (2005).
Xu, J. et al. Future of the particle replication in nonwetting templates (PRINT) technology. Angew. Chem. Int. Ed. 52, 6580–6589 (2013).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Champion, J. A. & Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA 103, 4930–4934 (2006).
Park, J. H. et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv. Mater. 20, 1630–1635 (2008).
Chauhan, V. P. et al. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. Int. Ed. 50, 11417–11420 (2011).
Gentile, F. et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J. Biomech. 41, 2312–2318 (2008).
Ruggiero, A. et al. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl Acad. Sci. USA 107, 12369–12374 (2010).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 121, 3–9 (2007).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627 (2010).
Alidori, S. et al. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Sci. Transl Med. 8, 331ra339 (2016).
Lacerda, L. et al. Carbon-nanotube shape and individualization critical for renal excretion. Small 4, 1130–1132 (2008).
Gustafson, H. H., Holt-Casper, D., Grainger, D. W. & Ghandehari, H. Nanoparticle uptake: the phagocyte problem. Nano Today 10, 487–510 (2015).
Verma, A. & Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 6, 12–21 (2010).
He, C., Hu, Y., Yin, L., Tang, C. & Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31, 3657–3666 (2010).
Yamamoto, Y., Nagasaki, Y., Kato, Y., Sugiyama, Y. & Kataoka, K. Long-circulating poly(ethylene glycol)–poly(D,L-lactide) block copolymer micelles with modulated surface charge. J. Control. Release 77, 27–38 (2001).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8, 129–138 (2009).
Davis, M. E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 6, 659–668 (2009).
Zhu, X. et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl Acad. Sci. USA 112, 7779–7784 (2015).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014).
Liang, X. et al. Short- and long-term tracking of anionic ultrasmall nanoparticles in kidney. ACS Nano 10, 387–395 (2016).
Satchell, S. C. & Braet, F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 296, F947–F956 (2009).
Curry, F. E. & Adamson, R. H. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann. Biomed. Eng. 40, 828–839 (2012).
Deen, W. M., Lazzara, M. J. & Myers, B. D. Structural determinants of glomerular permeability. Am. J. Physiol. Renal Physiol. 281, F579–F596 (2001).
Lal, M. A., Young, K. W. & Andag, U. Targeting the podocyte to treat glomerular kidney disease. Drug Discov. Today 20, 1228–1234 (2015).
Wu, H. Y. et al. Diagnostic performance of random urine samples using albumin concentration versus ratio of albumin to creatinine for microalbuminuria v screening in patients with diabetes mellitus: a systematic review and meta-analysis. JAMA Intern. Med. 174, 1108–1115 (2014).
Dahlman, J. E. et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 9, 648–655 (2014).
Dritschilo, A. et al. Phase I study of liposome-encapsulated c-raf antisense oligodeoxyribonucleotide infusion in combination with radiation therapy in patients with advanced malignancies. Clin. Cancer Res. 12, 1251–1259 (2006).
Elazar, V. et al. Sustained delivery and efficacy of polymeric nanoparticles containing osteopontin and bone sialoprotein antisenses in rats with breast cancer bone metastasis. Int. J. Cancer 126, 1749–1760 (2010).
Asgeirsdottir, S. A. et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am. J. Physiol. Renal Physiol. 294, F554–F561 (2008).
Groffen, A. J. et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J. Histochem. Cytochem. 46, 19–27 (1998).
Hironaka, K., Makino, H., Yamasaki, Y. & Ota, Z. Pores in the glomerular basement membrane revealed by ultrahigh-resolution scanning electron microscopy. Nephron 64, 647–649 (1993).
Suleiman, H. et al. Nanoscale protein architecture of the kidney glomerular basement membrane. eLife 2, e01149 (2013).
Kashtan, C. E. Alport syndrome & thin basement membrane nephropathy. GeneReviews https://www.ncbi.nlm.nih.gov/books/NBK1207/ (updated 25 Nov 2015).
Mohney, B. G. et al. A novel mutation of LAMB2 in a multigenerational mennonite family reveals a new phenotypic variant of Pierson syndrome. Ophthalmology 118, 1137–1144 (2011).
Kamaly, N. et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc. Natl Acad. Sci. USA 110, 6506–6511 (2013).
Zuckerman, J. E., Choi, C. H., Han, H. & Davis, M. E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl Acad. Sci. USA 109, 3137–3142 (2012).
Tryggvason, K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J. Am. Soc. Nephrol. 10, 2440–2445 (1999).
Tassin, M. T., Beziau, A., Gubler, M. C. & Boyer, B. Spatiotemporal expression of molecules associated with junctional complexes during the in vivo maturation of renal podocytes. Int. J. Dev. Biol. 38, 45–54 (1994).
Mallipattu, S. K. & He, J. C. The podocyte as a direct target for treatment of glomerular disease? Am. J. Physiol. Renal Physiol. 311, F46–F51 (2016).
Visweswaran, G. R. et al. Targeting rapamycin to podocytes using a vascular cell adhesion molecule-1 (VCAM-1)-harnessed SAINT-based lipid carrier system. PLoS ONE 10, e0138870 (2015).
Chiang, W. C. et al. Establishment of protein delivery systems targeting podocytes. PLoS ONE 5, e11837 (2010).
Chaudhury, C. et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 197, 315–322 (2003).
Wei, C. et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 14, 55–63 (2008).
Maile, L. A. et al. Blocking ligand occupancy of the αVβ3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology 155, 4665–4675 (2014).
Maile, L. A. et al. Blocking αVβ3 integrin ligand occupancy inhibits the progression of albuminuria in diabetic rats. J. Diabetes Res. 2014, 421827 (2014).
Pollinger, K. et al. Kidney podocytes as specific targets for cyclo(RGDfC)-modified nanoparticles. Small 8, 3368–3375 (2012).
Zuckerman, J. E., Gale, A., Wu, P., Ma, R. & Davis, M. E. siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Ther. 25, 53–64 (2015).
Ramos, A. M. et al. Designing drugs that combat kidney damage. Expert Opin. Drug Discov. 10, 541–556 (2015).
Falke, L. L., Gholizadeh, S., Goldschmeding, R., Kok, R. J. & Nguyen, T. Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 11, 233–244 (2015).
Morishita, Y. et al. siRNAs targeted to Smad4 prevent renal fibrosis in vivo. Sci. Rep. 4, 6424 (2014).
Zheng, X. et al. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation 82, 1781–1786 (2006).
Williams, R. M. et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 15, 2358–2364 (2015).
Dolman, M. E. et al. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int. J. Nanomedicine 7, 417–433 (2012).
Morishita, Y. et al. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int. J. Nanomedicine 10, 3475–3488 (2015).
Pascual, D. & Borque, A. Epidemiology of kidney cancer. Adv. Urol. 2008, 782381 (2008).
Koshkina, N. V. et al. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin. Cancer Res. 7, 3258–3262 (2001).
Boorjian, S. A. et al. Phase 1/2 clinical trial of interferon α2b and weekly liposome-encapsulated all-trans retinoic acid in patients with advanced renal cell carcinoma. J. Immunother. 30, 655–662 (2007).
Kulkarni, A. A., Vijaykumar, V. E., Natarajan, S. K., Sengupta, S. & Sabbisetti, V. S. Sustained inhibition of cMET–VEGFR2 signaling using liposome-mediated delivery increases efficacy and reduces toxicity in kidney cancer. Nanomedicine 12, 1853–1861 (2016).
Liu, J. et al. Comparison of sorafenib-loaded poly (lactic/glycolic) acid and DPPC liposome nanoparticles in the in vitro treatment of renal cell carcinoma. J. Pharm. Sci. 104, 1187–1196 (2015).
Takaha, N. et al. Significant induction of apoptosis in renal cell carcinoma cells transfected with cationic multilamellar liposomes containing the human interferon-β gene through activation of the intracellular type 1 interferon signal pathway. Int. J. Oncol. 40, 1441–1446 (2012).
Umebayashi, M., Makizono, T., Ichihara, H., Matsumoto, Y. & Ueoka, R. Inhibitory effects of cationic hybrid liposomes on the growth of human renal cell carcinoma. Anticancer Res. 30, 327–337 (2010).
Umebayashi, M., Matsumoto, Y. & Ueoka, R. Inhibitory effects of three-component hybrid liposomes containing cationic lipids without drugs on the growth of human renal tumor cells in vitro. Biol. Pharm. Bull. 31, 1816–1817 (2008).
Yamamoto, K. et al. Significant antitumor activity of cationic multilamellar liposomes containing human interferon-β gene in combination with 5-fluorouracil against human renal cell carcinoma. Int. J. Oncol. 33, 565–571 (2008).
Nakanishi, H. et al. Significant antitumoral activity of cationic multilamellar liposomes containing human IFN-β gene against human renal cell carcinoma. Clin. Cancer Res. 9, 1129–1135 (2003).
Skubitz, K. M. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in renal cell cancer. Invest. New Drugs 20, 101–104 (2002).
Tian, J. Q. et al. In vitro enhanced cytotoxicity of tumor-infiltrating lymphocytes transfected with tumor necrosis factor-related apoptosis-inducing ligand and/or interleukin-2 gene in human renal cell carcinoma. Urology 67, 1093–1098 (2006).
Akita, H. et al. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J. Control. Release 200, 97–105 (2015).
Yalcin, M. et al. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 29, 3825–3831 (2009).
Kasenda, B., Larkin, J. & Gore, M. Immunotherapies in early and advanced renal cell cancer. Prog. Tumor Res. 42, 1–10 (2015).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Gerlowski, L. E. & Jain, R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 31, 288–305 (1986).
Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 91, 3–6 (2015).
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl Med. 7, 314ra183 (2015).
Ramanathan, R. K. et al. Pilot study in patients with advanced solid tumors to evaluate feasibility of ferumoxytol (FMX) as tumor imaging agent prior to MM398, a nanoliposomal irinotecan (nalIRI) [abstract]. Cancer Res. 74 (19 Suppl.), CT224 (2014).
Koukourakis, M. I. et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J. Clin. Oncol. 17, 3512–3521 (1999).
Arrieta, O. et al. High liposomal doxorubicin tumour tissue distribution, as determined by radiopharmaceutical labelling with 99mTc-LD, is associated with the response and survival of patients with unresectable pleural mesothelioma treated with a combination of liposomal doxorubicin and cisplatin. Cancer Chemother. Pharmacol. 74, 211–215 (2014).
Grenier, N., Merville, P. & Combe, C. Radiologic imaging of the renal parenchyma structure and function. Nat. Rev. Nephrol. 12, 348–359 (2016).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Wilmer, M. J. et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 34, 156–170 (2016).
Jang, K. J. et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. (Camb.) 5, 1119–1129 (2013).
Jang, K. J. & Suh, K. Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10, 36–42 (2010).
Snouber, L. C. et al. Analysis of transcriptomic and proteomic profiles demonstrates improved Madin–Darby canine kidney cell function in a renal microfluidic biochip. Biotechnol. Prog. 28, 474–484 (2012).
Zhou, M., Ma, H., Lin, H. & Qin, J. Induction of epithelial-to-mesenchymal transition in proximal tubular epithelial cells on microfluidic devices. Biomaterials 35, 1390–1401 (2014).
Kim, S. & Takayama, S. Organ-on-a-chip and the kidney. Kidney Res. Clin. Pract. 34, 165–169 (2015).
Adler, M. et al. A quantitative approach to screen for nephrotoxic compounds in vitro. J. Am. Soc. Nephrol. 27, 1015–1028 (2016).
Kim, S. et al. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication 8, 015021 (2016).
Mae, S. et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 4, 1367 (2013).
Schmidt-Ott, K. M. How to grow a kidney: patient-specific kidney organoids come of age. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfw256 (2016).
Li, Z. et al. 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell 19, 516–529 (2016).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Takasato, M. & Little, M. H. A strategy for generating kidney organoids: recapitulating the development in human pluripotent stem cells. Dev. Biol. http://dx.doi.org/10.1016/j.ydbio.2016.08.024 (2016).
Takasato, M. et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126 (2014).
Reininger-Mack, A., Thielecke, H. & Robitzki, A. A. 3D-biohybrid systems: applications in drug screening. Trends Biotechnol. 20, 56–61 (2002).
Astashkina, A. I. et al. Nanoparticle toxicity assessment using an in vitro 3D kidney organoid culture model. Biomaterials 35, 6323–6331 (2014).
Astashkina, A. I., Mann, B. K., Prestwich, G. D. & Grainger, D. W. A. 3D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials 33, 4700–4711 (2012).
Valencia, P. M. et al. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 7, 10671–10680 (2013).
Kamaly, N. et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano 10, 5280–5292 (2016).
Karnik, R. et al. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 8, 2906–2912 (2008).
Valencia, P. M. et al. Single-step assembly of homogenous lipid-polymeric and lipid-quantum dot nanoparticles enabled by microfluidic rapid mixing. ACS Nano 4, 1671–1679 (2010).
Rhee, M. et al. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv. Mater. 23, H79–H83 (2011).
Chen, D. et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 134, 6948–6951 (2012).
Kim, Y. et al. Mass production and size control of lipid–polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 12, 3587–3591 (2012).
Lim, J. M. et al. Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer. ACS Nano 8, 6056–6065 (2014).
Zuckerman, J. E. et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl Acad. Sci. USA 111, 11449–11454 (2014).
Eliasof, S. et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl Acad. Sci. USA 110, 15127–15132 (2013).
Kohan, D. E. Kidney cell-specific knockdown: anything but simple. Am. J. Physiol. Renal Physiol. 309, F1007–F1008 (2015).
de Caestecker, M. et al. Bridging translation by improving preclinical study design in AKI. J. Am. Soc. Nephrol. 26, 2905–2916 (2015).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00617981?term (2014).
Escudier, B. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl Med. 3, 10 (2005).
Acknowledgements
O.C.F acknowledges support from the NHLBI HL127464, NCI CA151884, NIBIB EB015419 and the David Koch-Prostate Cancer Foundation Award in Nanotherapeutics. J.C.H is supported by NIH 1R01DK078897, NIH 1R01DK088541, NIH P01-DK-56492, Chinese 973 fund 2012CB517601.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of the article's content, writing, and review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
O.C.F. has financial interests in Selecta Biosciences, Tarveda Therapeutics, and Placon Therapeutics. D.A.A serves on the Board of Directors of Pfizer, Alnylam Pharmaceuticals, Seres Therapeutics, Tarveda Therapeutics and Placon Therapeutics. The other authors declare no competing interests.
Glossary
- Nanoparticles
-
Nanoscale particles with modifiable shape and charge capable of carrying a specific payload (drugs, diagnostic molecules etc.).
- Colloid
-
Substance formed by a non-crystalline material (here nanomaterial) of either natural or synthetic origin dispersed in a solution.
- Organ-on-a-chip
-
Microfluidic devices used to culture living cells under continuous perfusion in micorometer-sized chambers; they are primarily used to reproduce the physiological functions of tissues and organs as closely as possible.
- PEGylation
-
Attachment of polyethers to the surface of nanoparticles in order to minimize unwanted interactions with their biological surroundings.
- Cmax
-
Maximal serum concentration of a drug or nanoparticle achievable after administration.
- Top-down fabrication method
-
Synthesis of a structure by etching or removal of material from a template or substrate to achieve specific shapes or sizes.
- Particle replication in non-wetting templates
-
Fabrication technique whereby a pre-particle solution is distributed in a mould with nanosized cavities to generate nanoparticles with precisely defined shape, size, composition and surface properties.
- Aspect ratio
-
Ratio of the width to the height of a nanoparticle.
- Enhanced permeability and retention (EPR) effect
-
Describes the accumulation and retention of colloidal nanoparticles within tumour tissue as a result of increased endothelial gap junction distances due to heterogeneous vessel formation and growth.
Rights and permissions
About this article
Cite this article
Kamaly, N., He, J., Ausiello, D. et al. Nanomedicines for renal disease: current status and future applications. Nat Rev Nephrol 12, 738–753 (2016). https://doi.org/10.1038/nrneph.2016.156
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.156
This article is cited by
-
Microneedles loaded with cerium-manganese oxide nanoparticles for targeting macrophages in the treatment of rheumatoid arthritis
Journal of Nanobiotechnology (2024)
-
Preparation and PET/CT imaging of implant directed 68Ga-labeled magnetic nanoporous silica nanoparticles
Journal of Nanobiotechnology (2023)
-
Omics are Getting Us Closer to Understanding IgA Nephropathy
Archivum Immunologiae et Therapiae Experimentalis (2023)
-
Potential Medicinal Value of Rhein for Diabetic Kidney Disease
Chinese Journal of Integrative Medicine (2023)
-
Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions
Cell & Bioscience (2022)