Abstract
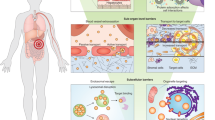
Kidney disease affects more than 10% of the global population and is associated with considerable morbidity and mortality, highlighting a need for new therapeutic options. Engineered nanoparticles for the treatment of kidney diseases (renal nanomedicines) represent one such option, enabling the delivery of targeted therapeutics to specific regions of the kidney. Although they are underdeveloped compared with nanomedicines for diseases such as cancer, findings from preclinical studies suggest that renal nanomedicines may hold promise. However, the physiological principles that govern the in vivo transport and interactions of renal nanomedicines differ from those of cancer nanomedicines, and thus a comprehensive understanding of these principles is needed to design nanomedicines that effectively and specifically target the kidney while ensuring biosafety in their future clinical translation. Herein, we summarize the current understanding of factors that influence the glomerular filtration, tubular uptake, tubular secretion and extrusion of nanoparticles, including size and charge dependency, and the role of specific transporters and processes such as endocytosis. We also describe how the transport and uptake of nanoparticles is altered by kidney disease and discuss strategic approaches by which nanoparticles may be harnessed for the detection and treatment of a variety of kidney diseases.
Key points
-
Despite considerable advances in cancer nanomedicines, renal nanomedicines for the treatment of kidney diseases are markedly underdeveloped.
-
The physiological principles that regulate the glomerular filtration, tubular secretion, luminal tubular uptake and re-elimination of nanoparticles in the kidneys may facilitate the selective targeting of nanoparticles to specific segments of the nephron.
-
Targeting of nanoparticles to different cell types in the glomerulus or to the glomerular basement membrane can be achieved through fine-tuning of their physicochemical properties based on our understanding of glomerular filtration and the glomerular filtration barrier.
-
Different transport pathways can be used to deliver nanoparticles to different components of the renal tubules, including the luminal and the basolateral sides of tubular epithelial cells and the interstitium.
-
Differences in nanoparticle transport and interactions between healthy and diseased kidney tissues offer opportunities for the design of nanoparticles that can selectively target specific kidney diseases.
-
A newly discovered organelle extrusion mechanism facilitates the elimination of endocytosed nanoparticles from the kidneys and may minimize the nephrotoxicity of future nanomedicines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kim, B. Y. S., Rutka, J. T. & Chan, W. C. W. Nanomedicine. N. Engl. J. Med. 363, 2434–2443 (2010).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Poon, W., Kingston, B. R., Ouyang, B., Ngo, W. & Chan, W. C. W. A framework for designing delivery systems. Nat. Nanotechnol. 15, 819–829 (2020).
Peng, C., Huang, Y. & Zheng, J. Renal clearable nanocarriers: overcoming the physiological barriers for precise drug delivery and clearance. J. Control. Rel. 322, 64–80 (2020).
Du, B., Yu, M. & Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 3, 358–374 (2018).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 1, 10–29 (2016).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, e10143 (2019).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update post COVID-19 vaccines. Bioeng. Transl. Med. 6, e10246 (2021).
Chauhan, V. P. & Jain, R. K. Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962 (2013).
Davis, M. E., Chen, Z. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 7, 771–782 (2008).
de Lázaro, I. & Mooney, D. J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 20, 1469–1479 (2021).
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Min, Y., Caster, J. M., Eblan, M. J. & Wang, A. Z. Clinical translation of nanomedicine. Chem. Rev. 115, 11147–11190 (2015).
de Boer, I. H. et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 98, S1–S115 (2020).
Levin, A. et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Breyer, M. D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug. Discov. 15, 568–588 (2016).
Kalantar-Zadeh, K., Jafar, T. H., Nitsch, D., Neuen, B. L. & Perkovic, V. Chronic kidney disease. Lancet 398, 786–802 (2021).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Prim. 3, 1–24 (2017).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179–c184 (2012).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380, 756–766 (2012).
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Sharma, K. et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 22, 1144–1151 (2011).
Amini, M., Salarifar, M., Amirbaigloo, A., Masoudkabir, F. & Esfahani, F. N-acetylcysteine does not prevent contrast-induced nephropathy after cardiac catheterization in patients with diabetes mellitus and chronic kidney disease: a randomized clinical trial. Trials 10, 45 (2009).
Ye, M., Lin, W., Zheng, J. & Lin, S. N-acetylcysteine for chronic kidney disease: a systematic review and meta-analysis. Am. J. Transl. Res. 13, 2472–2485 (2021).
Barr, L. F. & Kolodner, K. N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit. Care Med. 36, 1427–1435 (2008).
Ho, K. M. & Morgan, D. J. Meta-analysis of N-acetylcysteine to prevent acute renal failure after major surgery. Am. J. Kidney Dis. 53, 33–40 (2009).
Mainra, R., Gallo, K. & Moist, L. Effect of N‐acetylcysteine on renal function in patients with chronic kidney disease. Nephrology 12, 510–513 (2007).
Sisillo, E. et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit. Care Med. 36, 81–86 (2008).
Stokman, G., Qin, Y., Rácz, Z., Hamar, P. & Price, L. S. Application of siRNA in targeting protein expression in kidney disease. Adv. Drug. Deliv. Rev. 62, 1378–1389 (2010).
Peek, J. L. & Wilson, M. H. Cell and gene therapy for kidney disease. Nat. Rev. Nephrol. 19, 451–462 (2023).
Hu, C.-M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Zhou, J., Krishnan, N., Jiang, Y., Fang, R. H. & Zhang, L. Nanotechnology for virus treatment. Nano Today 36, 101031 (2021).
Pison, U., Welte, T., Giersig, M. & Groneberg, D. A. Nanomedicine for respiratory diseases. Eur. J. Pharmacol. 533, 341–350 (2006).
Goldsmith, M., Abramovitz, L. & Peer, D. Precision nanomedicine in neurodegenerative diseases. ACS Nano 8, 1958–1965 (2014).
Godin, B. et al. Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol. Sci. 31, 199–205 (2010).
Veiseh, O., Tang, B. C., Whitehead, K. A., Anderson, D. G. & Langer, R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug. Discov. 14, 45–57 (2015).
Kamaly, N., He, J. C., Ausiello, D. A. & Farokhzad, O. C. Nanomedicines for renal disease: current status and future applications. Nat. Rev. Nephrol. 12, 738–753 (2016).
Williams, R. M., Jaimes, E. A. & Heller, D. A. Nanomedicines for kidney diseases. Kidney Int. 90, 740–745 (2016).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Soo Choi, H. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Kunwar, A. et al. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 1780, 673–679 (2008).
Huang, Y., Yu, M. & Zheng, J. Proximal tubules eliminate endocytosed gold nanoparticles through an organelle-extrusion-mediated self-renewal mechanism. Nat. Nanotechnol. 18, 637–646 (2023).
Tsoi, K. M. et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 15, 1212–1221 (2016).
Satchell, S. C. & Braet, F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Ren. Physiol. 296, F947–F956 (2009).
Avasthi, P. S., Evan, A. P. & Hay, D. Glomerular endothelial cells in uranyl nitrate-induced acute renal failure in rats. J. Clin. Invest. 65, 121–127 (1980).
Lea, P. J., Silverman, M., Hegele, R. & Hollenberg, M. J. Tridimensional ultrastructure of glomerular capillary endothelium revealed by high-resolution scanning electron microscopy. Microvasc. Res. 38, 296–308 (1989).
Bearer, E. L. & Orci, L. Endothelial fenestral diaphragms: a quick-freeze, deep-etch study. J. Cell Biol. 100, 418–428 (1985).
Jeansson, M. & Haraldsson, B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am. J. Physiol. Ren. Physiol. 290, F111–F116 (2006).
Singh, A. et al. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J. Am. Soc. Nephrol. 18, 2885–2893 (2007).
Naylor, R. W., Morais, M. R. P. T. & Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 17, 112–127 (2021).
Miner, J. H. The glomerular basement membrane. Exp. Cell Res. 318, 973–978 (2012).
Miner, J. H. Renal basement membrane components. Kidney Int. 56, 2016–2024 (1999).
Palassini, M. & Remuzzi, A. Numerical analysis of viscous flow through fibrous media: a model for glomerular basement membrane permeability. Am. J. Physiol. Ren. Physiol. 274, F223–F231 (1998).
Hironaka, K. et al. Ultrastructural change of the glomerular basement membrane in rats with Heymann nephritis revealed by ultrahigh resolution scanning electron microscopy. J. Pathol. 179, 112–120 (1996).
Hironaka, K., Makino, H., Yamasaki, Y. & Ota, Z. Renal basement membranes by ultrahigh resolution scanning electron microscopy. Kidney Int. 43, 334–345 (1993).
Hironaka, K., Makino, H., Yamasaki, Y. & Ota, Z. Pores in the glomerular basement membrane revealed by ultrahigh-resolution scanning electron microscopy. Nephron 64, 647–649 (1993).
Gagliardini, E., Conti, S., Benigni, A., Remuzzi, G. & Remuzzi, A. Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J. Am. Soc. Nephrol. 21, 2081–2089 (2010).
Choi, C. H. J., Zuckerman, J. E., Webster, P. & Davis, M. E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl Acad. Sci. USA 108, 6656–6661 (2011).
Zuckerman, J. E., Choi, C. H. J., Han, H. & Davis, M. E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl Acad. Sci. USA 109, 3137–3142 (2012).
Rennke, H. G. & Venkatachalam, M. A. Glomerular permeability: In vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 11, 44–53 (1977).
Arturson, G. & Wallenius, G. The renal clearance of dextran of different molecular sizes in normal humans. Scand. J. Clin. Lab. Invest. 16, 81–86 (1964).
Zhou, C., Long, M., Qin, Y., Sun, X. & Zheng, J. Luminescent gold nanoparticles with efficient renal clearance. Angew. Chem. Int. Ed. Engl. 50, 3168–3172 (2011).
Zhou, C. et al. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew. Chem. Int. Ed. Engl. 51, 10118–10122 (2012).
Yu, M., Xu, J. & Zheng, J. Renal clearable luminescent gold nanoparticles: from the bench to the clinic. Angew. Chem. Int. Ed. Engl. 58, 4112–4128 (2019).
Yang, S. et al. Renal clearance and degradation of glutathione-coated copper nanoparticles. Bioconjug Chem. 26, 511–519 (2015).
Tang, S., Huang, Y. & Zheng, J. Salivary excretion of renal-clearable silver nanoparticles. Angew. Chem. Int. Ed. Engl. 59, 19894–19898 (2020).
Tang, S. et al. Tailoring renal clearance and tumor targeting of ultrasmall metal nanoparticles with particle density. Angew. Chem. Int. Ed. Engl. 55, 16039–16043 (2016).
Xie, M. et al. Brain tumor imaging and delivery of sub-5 nm magnetic iron oxide nanoparticles in an orthotopic murine model of glioblastoma. ACS Appl. Nano Mater. 5, 9706–9718 (2022).
Huang, J. et al. Facile non-hydrothermal synthesis of oligosaccharide coated sub-5 nm magnetic iron oxide nanoparticles with dual MRI contrast enhancement effects. J. Mater. Chem. B 2, 5344–5351 (2014).
Wei, H. et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl Acad. Sci. USA 114, 2325–2330 (2017).
Burns, A. A. et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 9, 442–448 (2009).
Lux, F. et al. Ultrasmall rigid particles as multimodal probes for medical applications. Angew. Chem. Int. Ed. Engl. 50, 12299–12303 (2011).
Lux, F. et al. AGuIX® from bench to bedside – transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 92, 20180365 (2019).
Kang, H. et al. Renal clearable organic nanocarriers for bioimaging and drug delivery. Adv. Mater. 28, 8162–8168 (2016).
Kang, H. et al. Renal clearable theranostic nanoplatforms for gastrointestinal stromal tumors. Adv. Mater. 32, 1905899 (2020).
Phillips, E. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 6, 260ra149 (2014).
Zanoni, D. K. et al. Use of ultrasmall core-shell fluorescent silica nanoparticles for image-guided sentinel lymph node biopsy in head and neck melanoma: a nonrandomized clinical trial. JAMA Netw. Open. 4, e211936 (2021).
Verry, C. et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 6, eaay5279 (2020).
Liu, Z. et al. An ultrasmall RuO2 nanozyme exhibiting multienzyme-like activity for the prevention of acute kidney injury. ACS Appl. Mater. Interfaces 12, 31205–31216 (2020).
Ni, D. et al. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 9, 5421 (2018).
Shin, T.-H. et al. High-resolution T1 MRI via renally clearable dextran nanoparticles with an iron oxide shell. Nat. Biomed. Eng. 5, 252–263 (2021).
Huang, J., Li, J., Lyu, Y., Miao, Q. & Pu, K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
Cheng, P. et al. Artificial urinary biomarkers for early diagnosis of acute renal allograft rejection. Angew. Chem. Int. Ed. Engl. 62, e202306539 (2023).
Whitley, M. J. et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 8, 320ra324 (2016).
Bugaj, J. E. & Dorshow, R. B. Pre-clinical toxicity evaluation of MB-102, a novel fluorescent tracer agent for real-time measurement of glomerular filtration rate. Regul. Toxicol. Pharmacol. 72, 26–38 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03686215 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05425719 (2023).
Du, B. et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 12, 1096–1102 (2017).
Du, B. et al. Tailoring kidney transport of organic dyes with low-molecular-weight PEGylation. Bioconjug. Chem. 31, 241–247 (2020).
Caulfield, J. P. & Farquhar, M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc. Natl Acad. Sci. USA 73, 1646–1650 (1976).
Kanwar, Y. S. & Farquhar, M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc. Natl Acad. Sci. USA 76, 1303–1307 (1979).
Bohrer, M. P. et al. Permselectivity of the glomerular capillary wall: facilitated filtration of circulating polycations. J. Clin. Invest. 61, 72–78 (1978).
Brenner, B. M., Hostetter, T. H. & Humes, H. D. Glomerular permselectivity: barrier function based on discrimination of molecular size and charge. Am. J. Physiol. Ren. Physiol. 234, F455–F460 (1978).
Chang, R. L. S., Deen, W. M., Robertson, C. R. & Brenner, B. M. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 8, 212–218 (1975).
Comper, W. D. & Glasgow, E. F. Charge selectivity in kidney ultrafiltration. Kidney Int. 47, 1242–1251 (1995).
Adal, Y., Pratt, L. & Comper, W. D. Transglomerular transport of DEAE dextran in the isolated perfused kidney. Microcirculation 1, 169–174 (1994).
Asgeirsson, D., Venturoli, D., Rippe, B. & Rippe, C. Increased glomerular permeability to negatively charged Ficoll relative to neutral Ficoll in rats. Am. J. Physiol. Ren. Physiol. 291, F1083–F1089 (2006).
Sirich, T. L., Aronov, P. A., Plummer, N. S., Hostetter, T. H. & Meyer, T. W. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 84, 585–590 (2013).
Anzai, N., Jutabha, P. & Endou, H. Molecular mechanism of ochratoxin a transport in the kidney. Toxins 2, 1381–1398 (2010).
Landersdorfer, C. B. et al. Competitive inhibition of renal tubular secretion of gemifloxacin by probenecid. Antimicrob. Agents Chemother. 53, 3902–3907 (2009).
Fritzberg, A. R., Kasina, S., Eshima, D. & Johnson, D. L. Synthesis and biological evaluation of technetium-99m MAG3 as a hippuran. Replacement. J. Nucl. Med. 27, 111–116 (1986).
Müller-Suur, R. & Müller-Suur, C. Glomerular filtration and tubular secretion of MAG-3 in the rat kidney. J. Nucl. Med. 30, 1986–1991 (1989).
Burckhardt, G., Bahn, A. & Wolff, N. A. Molecular physiology of renal p-aminohippurate secretion. Physiol 16, 114–118 (2001).
Du, B. et al. Hyperfluorescence imaging of kidney cancer enabled by renal secretion pathway dependent efflux transport. Angew. Chem. Int. Ed. Engl. 60, 351–359 (2021).
Alander, J. T. et al. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 940585 (2012).
Carr, J. A. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl Acad. Sci. USA 115, 4465–4470 (2018).
Tahara, H. et al. Inhibition of OAT3-mediated renal uptake as a mechanism for drug-drug interaction between fexofenadine and probenecid. Drug. Metab. Dispos. 34, 743–747 (2006).
Naumenko, V. et al. Intravital microscopy reveals a novel mechanism of nanoparticles excretion in kidney. J. Control. Rel. 307, 368–378 (2019).
Wyss, P. P. et al. Renal clearance of polymeric nanoparticles by mimicry of glycan surface of viruses. Biomaterials 230, 119643 (2020).
Williams, R. M. et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 15, 2358–2364 (2015).
Williams, R. M. et al. Selective nanoparticle targeting of the renal tubules. Hypertension 71, 87–94 (2018).
Kurtzman, N. A. & Pillay, V. K. G. Renal reabsorption of glucose in health and disease. Arch. Intern. Med. 131, 901–904 (1973).
Russo, L. M. et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 71, 504–513 (2007).
Tenten, V. et al. Albumin is recycled from the primary urine by tubular transcytosis. J. Am. Soc. Nephrol. 24, 1966–1980 (2013).
Cheng, P. & Pu, K. Molecular imaging and disease theranostics with renal-clearable optical agents. Nat. Rev. Mater. 6, 1095–1113 (2021).
Sancey, L. et al. Long-term in vivo clearance of gadolinium-based AGuIX nanoparticles and their biocompatibility after systemic injection. ACS Nano 9, 2477–2488 (2015).
He, X.-K., Yuan, Z.-X., Wu, X.-J., Xu, C.-Q. & Li, W.-Y. Low molecular weight hydroxyethyl chitosan-prednisolone conjugate for renal targeting therapy: synthesis, characterization and in vivo studies. Theranostics 2, 1054–1063 (2012).
Liu, D. et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 6, eabb7422 (2020).
Matsuura, S. et al. l-Serine–modified polyamidoamine dendrimer as a highly potent renal targeting drug carrier. Proc. Natl Acad. Sci. USA 115, 10511–10516 (2018).
Xie, D. et al. Kidney-targeted delivery of prolyl hydroxylase domain protein 2 small interfering RNA with nanoparticles alleviated renal ischemia/reperfusion injury. J. Pharmacol. Exp. Ther. 378, 235–243 (2021).
Oroojalian, F. et al. Efficient megalin targeted delivery to renal proximal tubular cells mediated by modified-polymyxin B-polyethylenimine based nano-gene-carriers. Mater. Sci. Eng. C Mater. Biol. Appl. 79, 770–782 (2017).
Kok, R. J., Haas, M., Moolenaar, F., de Zeeuw, D. & Meijer, D. K. Drug delivery to the kidneys and the bladder with the low molecular weight protein lysozyme. Ren. Fail. 20, 211–217 (1998).
Zhang, Z. et al. The targeting of 14-succinate triptolide-lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterials 30, 1372–1381 (2009).
Bidwell, I. I. I. et al. A kidney-selective biopolymer for targeted drug delivery. Am. J. Physiol. Ren. Physiol. 312, F54–F64 (2017).
Chen, Y. et al. A promising NIR‐II fluorescent sensor for peptide‐mediated long‐term monitoring of kidney dysfunction. Angew. Chem. Int. Ed. Engl. 133, 15943–15949 (2021).
Wang, J. et al. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 11, 5584–5595 (2018).
Franssen, E. J. F., Moolenaar, F., de Zeeuw, D. & Meijer, D. K. F. Drug targeting to the kidney with low-molecular-weight proteins. Adv. Drug. Deliv. Rev. 14, 67–88 (1994).
Ordikhani, F. et al. Selective trafficking of light chain-conjugated nanoparticles to the kidney and renal cell carcinoma. Nano Today 35, 100990 (2020).
Yamamoto, Y. et al. Poly(vinylpyrrolidone-co-dimethyl maleic acid) as a novel renal targeting carrier. J. Control. Rel. 95, 229–237 (2004).
Kamada, H. et al. Synthesis of a poly(vinylpyrrolidone-co-dimethyl maleic anhydride) co-polymer and its application for renal drug targeting. Nat. Biotechnol. 21, 399–404 (2003).
Jia, Z. et al. Micelle-forming dexamethasone prodrug attenuates nephritis in lupus-prone mice without apparent glucocorticoid side effects. ACS Nano 12, 7663–7681 (2018).
Dolman, M. E. M., Harmsen, S., Storm, G., Hennink, W. E. & Kok, R. J. Drug targeting to the kidney: advances in the active targeting of therapeutics to proximal tubular cells. Adv. Drug. Deliv. Rev. 62, 1344–1357 (2010).
Andersson, M., Nilsson, U., Hjalmarsson, C., Haraldsson, B. & Nyström, J. S. Mild renal ischemia-reperfusion reduces charge and size selectivity of the glomerular barrier. Am. J. Physiol. Ren. Physiol. 292, F1802–F1809 (2007).
Finch, N. C. et al. Reduced glomerular filtration in diabetes is attributable to loss of density and increased resistance of glomerular endothelial cell fenestrations. J. Am. Soc. Nephrol. 33, 1120–1136 (2022).
Rippe, C., Rippe, A., Larsson, A., Asgeirsson, D. & Rippe, B. Nature of glomerular capillary permeability changes following acute renal ischemia-reperfusion injury in rats. Am. J. Physiol. Ren. Physiol. 291, F1362–F1368 (2006).
Floege, J. & Feehally, J. in Comprehensive Clinical Nephrology (eds Floege, J., Johnson, R. J. & Feehally, J.) 193–207 (Mosby, 2010).
Vaden, S. L. Glomerular disease. Top. Companion Anim. Med. 26, 128–134 (2011).
Avraham, S., Korin, B., Chung, J.-J., Oxburgh, L. & Shaw, A. S. The mesangial cell – the glomerular stromal cell. Nat. Rev. Nephrol. 17, 855–864 (2021).
Scindia, Y. M., Deshmukh, U. S. & Bagavant, H. Mesangial pathology in glomerular disease: targets for therapeutic intervention. Adv. Drug. Deliv. Rev. 62, 1337–1343 (2010).
Guo, L. et al. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 8, 878 (2017).
Tuffin, G., Waelti, E., Huwyler, J., Hammer, C. & Marti, H.-P. Immunoliposome targeting to mesangial cells: a promising strategy for specific drug delivery to the kidney. J. Am. Soc. Nephrol. 16, 3295–3305 (2005).
Zuckerman, J. E., Gale, A., Wu, P., Ma, R. & Davis, M. E. siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid. Ther. 25, 53–64 (2015).
Suh, J. H. & Miner, J. H. The glomerular basement membrane as a barrier to albumin. Nat. Rev. Nephrol. 9, 470–477 (2013).
Cohen, S., Vernier, R. & Michael, A. The effect of charge on the renal distribution of ferritin. Am. J. Pathol. 110, 170–181 (1983).
Bennett, K. M., Bertram, J. F., Beeman, S. C. & Gretz, N. The emerging role of MRI in quantitative renal glomerular morphology. Am. J. Physiol. Ren. Physiol. 304, F1252–F1257 (2013).
Bennett, K. M. et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn. Reson. Med. 60, 564–574 (2008).
Leeuwis, J. W., Nguyen, T. Q., Dendooven, A., Kok, R. J. & Goldschmeding, R. Targeting podocyte-associated diseases. Adv. Drug. Deliv. Rev. s. 62, 1325–1336 (2010).
Wu, L. et al. Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. Int. J. Mol. Med. 39, 851–860 (2017).
Pollinger, K. et al. Kidney podocytes as specific targets for cyclo (RGDfC)‐modified nanoparticles. Small 8, 3368–3375 (2012).
Hauser, P. V. et al. Novel siRNA delivery system to target podocytes in vivo. PLoS ONE 5, e9463 (2010).
Chevalier, R. L. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 311, F145–F161 (2016).
Yu, M., Liu, J., Ning, X. & Zheng, J. High-contrast noninvasive imaging of kidney clearance kinetics enabled by renal clearable nanofluorophores. Angew. Chem. Int. Ed. Engl. 54, 15434–15438 (2015).
Yu, M. et al. Noninvasive staging of kidney dysfunction enabled by renal-clearable luminescent gold nanoparticles. Angew. Chem. Int. Ed. Engl. 55, 2787–2791 (2016).
Xu, J. et al. In vivo X-ray imaging of transport of renal clearable gold nanoparticles in the kidneys. Angew. Chem. Int. Ed. Engl. 56, 13356–13360 (2017).
Chen, Q. et al. Nanodrugs alleviate acute kidney injury: manipulate RONS at kidney. Bioact. Mater. 22, 141–167 (2023).
Chen, W. & Li, D. Reactive oxygen species (ROS)-responsive nanomedicine for solving ischemia-reperfusion injury. Front. Chem. 8, 732 (2020).
Feng, S. et al. Novel gold-platinum nanoparticles serve as broad-spectrum antioxidants for attenuating ischemia reperfusion injury of the kidney. Kidney Int. 102, 1057–1072 (2022).
Hou, J. et al. Treating acute kidney injury with antioxidative black phosphorus nanosheets. Nano Lett. 20, 1447–1454 (2020).
Jiang, D. et al. Nanomedicines for renal management: from imaging to treatment. Acc. Chem. Res. 53, 1869–1880 (2020).
Jiang, D. et al. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat. Biomed. Eng. 2, 865–877 (2018).
Liu, T. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11, 2788 (2020).
Yu, H. et al. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials 219, 119368 (2019).
Qin, S., Wu, B., Gong, T., Zhang, Z.-R. & Fu, Y. Targeted delivery via albumin corona nanocomplex to renal tubules to alleviate acute kidney injury. J. Control. Rel. 349, 401–412 (2022).
Wang, S. et al. Selenium nanoparticles alleviate ischemia reperfusion injury-induced acute kidney injury by modulating GPx-1/NLRP3/caspase-1 pathway. Theranostics 12, 3882–3895 (2022).
Tang, T.-T. et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 6, eaaz0748 (2020).
Liu, Z. et al. Neutrophil membrane-enveloped nanoparticles for the amelioration of renal ischemia-reperfusion injury in mice. Acta Biomater. 104, 158–166 (2020).
Deng, X. et al. Kidney-targeted triptolide-encapsulated mesoscale nanoparticles for high-efficiency treatment of kidney injury. Biomater. Sci. 7, 5312–5323 (2019).
Vallorz, E. L., Blohm-Mangone, K., Schnellmann, R. G. & Mansour, H. M. Formoterol PLGA-PEG nanoparticles induce mitochondrial biogenesis in renal proximal tubules. AAPS J. 23, 88 (2021).
Vallorz, E. L., Janda, J., Mansour, H. M. & Schnellmann, R. G. Kidney targeting of formoterol containing polymeric nanoparticles improves recovery from ischemia reperfusion-induced acute kidney injury in mice. Kidney Int. 102, 1073–1089 (2022).
Han, S. J. et al. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 98, 76–87 (2020).
Guo, X. et al. Kidney-targeted renalase agonist prevents cisplatin-induced chronic kidney disease by inhibiting regulated necrosis and inflammation. J. Am. Soc. Nephrol. 33, 342–356 (2022).
Williams, R. M. et al. Kidney-targeted redox scavenger therapy prevents cisplatin-induced acute kidney injury. Front. Pharmacol. 12, 790913 (2022).
Kaissling, B. & Le Hir, M. The renal cortical interstitium: morphological and functional aspects. Histochem. Cell Biol. 130, 247–262 (2008).
Zeisberg, M. & Kalluri, R. Physiology of the renal interstitium. Clin. J. Am. Soc. Nephrol. 10, 1831–1840 (2015).
Perazella, M. A. & Markowitz, G. S. Drug-induced acute interstitial nephritis. Nat. Rev. Nephrol. 6, 461–470 (2010).
Humphreys, B. D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 80, 309–326 (2018).
Tan, L. et al. Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models. Open. Chem. 18, 1207–1216 (2020).
Zhu, X.-Y. et al. Targeted imaging of renal fibrosis using antibody-conjugated gold nanoparticles in renal artery stenosis. Invest. Radiol. 53, 623–628 (2018).
Li, R. et al. Targeted delivery of celastrol to renal interstitial myofibroblasts using fibronectin-binding liposomes attenuates renal fibrosis and reduces systemic toxicity. J. Control. Rel. 320, 32–44 (2020).
Cheng, H.-T. et al. Delivery of sorafenib by myofibroblast-targeted nanoparticles for the treatment of renal fibrosis. J. Control. Rel. 346, 169–179 (2022).
Oh, N. & Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 9, 51–63 (2014).
Ho, L. W. C., Yin, B., Dai, G. & Choi, C. H. J. Effect of surface modification with hydrocarbyl groups on the exocytosis of nanoparticles. Biochemistry 60, 1019–1030 (2021).
Ho, L. W. C. et al. Mammalian cells exocytose alkylated gold nanoparticles via extracellular vesicles. ACS Nano 16, 2032–2045 (2022).
Chithrani, B. D. & Chan, W. C. W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7, 1542–1550 (2007).
Kim, C. et al. Regulating exocytosis of nanoparticles via host–guest chemistry. Org. Biomol. Chem. 13, 2474–2479 (2015).
Balfourier, A. et al. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl Acad. Sci. USA 117, 103–113 (2020).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Gong, N., Sheppard, N. C., Billingsley, M. M., June, C. H. & Mitchell, M. J. Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 16, 25–36 (2021).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Acknowledgements
The authors acknowledge support from the National Institutes of Health (NIH) (R01DK124881 (J.Z), R01DK115986 (J.Z), R01DK126140 (M.Y.)), and the Distinguished Chair of Natural Sciences & Mathematics (to J.Z.) from The University of Texas at Dallas.
Author information
Authors and Affiliations
Contributions
Y.H. and J.Z. researched data for the article. Y.H., X.N., S.A., M.Y. and J.Z. contributed substantially to discussion of the content. Y.H., M.Y. and J.Z. wrote the article. Y.H., Q.C., N.R., R.S., M.Y. and J.Z. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Reza Abdi, who co-reviewed with Vivek Kasinath, Kanyi Pu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.’
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Ning, X., Ahrari, S. et al. Physiological principles underlying the kidney targeting of renal nanomedicines. Nat Rev Nephrol (2024). https://doi.org/10.1038/s41581-024-00819-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-024-00819-z