Key Points
-
Directing the differentiation of stem cells to kidney tissues requires an understanding of kidney morphogenesis
-
The kidney is a mesodermal organ and hence is derived from the primitive streak
-
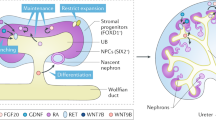
The primitive streak-derived intermediate mesoderm gives rise to both the ureteric bud and the metanephric mesenchyme
-
The anterior intermediate mesoderm forms the mesonephric duct, which gives rise to the ureteric bud whereas the posterior intermediate mesoderm gives rise to the metanephric mesenchyme; both regions are required to recreate the entire kidney
-
Using their understanding of kidney development, a number of groups have developed approaches to generate nephrons or whole kidney organoids from human pluripotent stem cells
-
Kidney tissues generated in vitro are now being investigated as tools for disease modelling, drug screening, cell therapy and bioengineering of replacement renal tissue
Abstract
The treatment of renal failure has seen little change in the past 70 years. Patients with end-stage renal disease (ESRD) are treated with renal replacement therapy, including dialysis or organ transplantation. The growing imbalance between the availability of donor organs and prevalence of ESRD is pushing an increasing number of patients to undergo dialysis. Although the prospect of new treatment options for patients through regenerative medicine has long been suggested, advances in the generation of human kidney cell types through the directed differentiation of human pluripotent stem cells over the past 2 years have brought this prospect closer to delivery. These advances are the result of careful research into mammalian embryogenesis. By understanding the decision points made within the embryo to pattern the kidney, it is now possible to recreate self-organizing kidney tissues in vitro. In this Review, we describe the key decision points in kidney development and how these decisions have been mimicked experimentally. Recreation of human nephrons from human pluripotent stem cells opens the door to patient-derived disease models and personalized drug and toxicity screening. In the long term, we hope that these efforts will also result in the generation of bioengineered organs for the treatment of kidney disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Hentze, H. et al. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2, 198–210 (2009).
Lin, S. A. et al. Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and renal progenitors. Stem Cells Dev. 19, 1637–1648 (2010).
Takahashi, K. et al. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Takasato, M. & Little, M. H. Generating a self-organising kidney from pluripotent cells. Curr. Opin. Organ. Transplant. 20, 178–186 (2015).
Takasato, M. et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126 (2014).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Mae, S.-I. et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 4, 1367 (2013).
Lam, A. Q. et al. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 25, 1211–1225 (2014).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Xia, Y. et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 15, 1507–1515 (2013).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Freedman, B. S. et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 (2015).
Tam, P. P. L. & Loebel, D. A. F. Gene function in mouse embryogenesis: get set for gastrulation. Nat. Rev. Genet. 8, 368–381 (2007).
Takaoka, K. & Hamada, H. Cell fate decisions and axis determination in the early mouse embryo. Development 139, 3–14 (2012).
Perea-Gomez, A. et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev. Cell 3, 745–756 (2002).
Ben-Haim, N. et al. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev. Cell 11, 313–323 (2006).
Funa, N. S. et al. β-Catenin regulates primitive streak induction through collaborative interactions with SMAD2/SMAD3 and OCT4. Cell Stem Cell 16, 639–652 (2015).
Liu, P. et al. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 22, 361–365 (1999).
Mizutani, A. et al. Cell type-specific target selection by combinatorial binding of Smad2/3 proteins and hepatocyte nuclear factor 4α in HepG2 cells. J. Biol. Chem. 286, 29848–29860 (2011).
Conlon, F. L. et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120, 1919–1928 (1994).
Lu, C. C. & Robertson, E. J. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev. Biol. 273, 149–159 (2004).
Bachiller, D. et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403, 658–661 (2000).
Winnier, G., Blessing, M., Labosky, P. A. & Hogan, B. L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9, 2105–2116 (1995).
Beppu, H. et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev. Biol. 221, 249–258 (2000).
Vincent, S. D., Dunn, N. R., Hayashi, S., Norris, D. P. & Robertson, E. J. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 17, 1646–1662 (2003).
Pereira, L. A., Wong, M. S., Mei Lim, S., Stanley, E. G. & Elefanty, A. G. The Mix family of homeobox genes-Key regulators of mesendoderm formation during vertebrate development. Dev. Biol. 367, 163–177 (2012).
Hart, A. H. et al. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development 129, 3597–3608 (2002).
Sumi, T., Tsuneyoshi, N., Nakatsuji, N. & Suemori, H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/β-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979 (2008).
Davis, R. P. et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 111, 1876–1884 (2008).
Burridge, P. W., Keller, G., Gold, J. D. & Wu, J. C. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10, 16–28 (2012).
Jackson, S. A., Schiesser, J., Stanley, E. G. & Elefanty, A. G. Differentiating embryonic stem cells pass through 'temporal windows' that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. PLoS ONE. 5, e10706 (2010).
Gertow, K. et al. WNT3A promotes hematopoietic or mesenchymal differentiation from hESCs depending on the time of exposure. Stem Cell Reports 1, 53–65 (2013).
Peng, G. et al. Spatial transcriptome for the molecular annotation of lineage fates and cell identity in mid-gastrula mouse embryo. Dev. Cell 36, 681–697 (2016).
James, R. G. & Schultheiss, T. M. Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev. Biol. 253, 109–124 (2003).
Sweetman, D., Wagstaff, L., Cooper, O., Weijer, C. & Münsterberg, A. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Dev. Biol. 8, 63 (2008).
Obara-Ishihara, T., Kuhlman, J., Niswander, L. & Herzlinger, D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126, 1103–1108 (1999).
James, R. G. & Schultheiss, T. M. Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev. Biol. 288, 113–125 (2005).
Wijgerde, M., Karp, S., McMahon, J. & McMahon, A. P. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev. Biol. 286, 149–157 (2005).
Biben, C. et al. Murine cerberus homologue mCer-1: a candidate anterior patterning molecule. Dev. Biol. 194, 135–151 (1998).
Fleming, B. M., Yelin, R., James, R. G. & Schultheiss, T. M. A role for Vg1/Nodal signaling in specification of the intermediate mesoderm. Development 140, 1819–1829 (2013).
Colvin, J. S., Feldman, B., Nadeau, J. H., Goldfarb, M. & Ornitz, D. M. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev. Dyn. 216, 72–88 (1999).
Little, M. H., & McMahon, A. P. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 4, a008300 (2012).
Xu, J. et al. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev. Cell 31, 434–447 (2014).
Takasato, M. & Little, M. H. The origin of the mammalian kidney: implications for recreating the kidney in vitro. Development 142, 1937–1947 (2015).
Deng, C., Lewandoski, M. & Pourquié, O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development 134, 4033–4041 (2007).
Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 (2008).
Sakai, Y. et al. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 15, 213–225 (2001).
Sajithlal, G., Zou, D., Silvius, D. & Xu, P. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev. Biol. 284, 323–336 (2005).
Dressler, G. R. Advances in early kidney specification, development and patterning. Development 136, 3863–3874 (2009).
Mugford, J. W., Sipilä, P., McMahon, J. A. & McMahon, A. P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 324, 88–98 (2008).
Bouchard, M., Souabni, A., Mandler, M., Neubüser, A. & Busslinger, M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 16, 2958–2970 (2002).
Kobayashi, A. et al. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132, 2809–2823 (2005).
Grote, D., Souabni, A., Busslinger, M. & Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133, 53–61 (2006).
Chi, X. et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199–209 (2009).
Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994).
Moore, M. W. et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382, 76–79 (1996).
Enomoto, H. et al. GFRα1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21, 317–324 (1998).
Costantini, F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip. Rev. Dev. Biol. 1, 693–713 (2012).
Costantini, F. & Kopan, R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698–712 (2010).
Majumdar, A., Vainio, S., Kispert, A., McMahon, J. & McMahon, A. P. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 (2003).
Lu, B. C. et al. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat. Genet. 41, 1295–1302 (2009).
Zhao, H. et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 276, 403–415 (2004).
Burn, S. F. et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev. Biol. 352, 288–298 (2011).
Marose, T. D., Merkel, C. E., McMahon, A. P., Carroll, T. J. β-Catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev. Biol. 314, 112–126 (2008).
Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. & McMahon, A. P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Yu, J. et al. Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136, 161–171 (2009).
Mendelsohn, C., Batourina, E., Fung, S., Gilbert, T. & Dodd, J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126, 1139–1148 (1999).
Batourina, E. et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat. Genet. 27, 74–78 (2001).
Rosselot, C. et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 (2010).
Caruana, G. et al. Spatial gene expression in the T-stage mouse metanephros. Gene Expr. Patterns 6, 807–825 (2006).
Song, B. et al. The directed differentiation of human iPS cells into kidney podocytes. PloS ONE 7, e46453 (2012).
Zhang, J. et al. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp. Nephrol. 121, e23–e27 (2012).
Vetter, M. R. & Gibley, C. W. Morphogenesis and histochemistry of the developing mouse kidney. J. Morphol. 120, 135–155 (1966).
Georgas, K. M., Chiu, H. S., Lesieur, E., Rumballe, B. A. & Little, M. H. Expression of metanephric nephron-patterning genes in differentiating mesonephric tubules. Dev. Dyn. 240, 1600–1612 (2011).
Woolf, A. S., Gnudi, L., Long, D. A., Roles of angiopoietins in kidney development and disease. J. Am. Soc. Nephrol. 20, 239–244 (2009).
Schoenwolf, G. Larsen's Human Embryology 5th Edition Ch. 3,4,15 (Churchill Livingstone, 2014).
Kobayashi, A. et al. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports 3, 650–662 (2014).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
Self, M. et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214–5228 (2006).
Boyle, S. et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234–245 (2008).
Cebrian, C., Asai, N., D'Agati, V. & Costantini, F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 7, 127–137 (2014).
Karner, C. M. et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011).
Barak, H. et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell 22, 1191–1207 (2010).
Dudley, A. T., Godin, R. E. & Robertson, E. J. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 13, 1601–1613 (1999).
Brown, A. C. et al. Role for compartmentalization in nephron progenitor differentiation. Proc. Natl. Acad. Sci. USA 110, 4640–4645 (2013).
Brown, A. C., Muthukrishnan, S. D. & Oxburgh, L. A synthetic niche for nephron progenitor cells. Dev. Cell 34, 229–241 (2015).
Park, J. S. et al. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev. Cell 23, 637–651 (2012).
Tanigawa, S. et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev. Biol. 352, 58–69 (2011).
Perantoni, A. O. et al. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132, 3859–3871 (2005).
Little, M. et al. Kidney development: two tales of tubulogenesis. Curr. Top. Dev. Biol. 90, 193–229 (2010).
Cheng, H. T. et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801–811 (2007).
Wingert, R. A. & Davidson, A. J. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev. Dyn. 240, 2011–2027 (2011).
Schneider, J., Arraf, A. A., Grinstein, M., Yelin, R. & Schultheiss, T. M. Wnt signaling orients the proximal-distal axis of chick kidney nephrons. Development 142, 2686–2695 (2015).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Sequeira-Lopez, M. L. et al. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R138–R149 (2015).
Hu, Y., Li, M., Göthert, J. R., Gomez, R. A. & Sequeira-Lopez, M. L. Hemovascular progenitors in the kidney require sphingosine-1-phosphate receptor 1 for vascular development. J. Am. Soc. Nephrol. 27, 1984–1995 (2016).
Xu, J., Nie, X., Cai, X., Cai, C. L. & Xu, P. X. Tbx18 is essential for normal development of vasculature network and glomerular mesangium in the mammalian kidney. Dev. Biol. 391, 17–31 (2014).
Sharmin, S. et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am. Soc. Nephrol. 27, 1778–1791 (2016).
Bedzhov, I. & Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032–1044 (2014).
Roost, M. S. et al. KeyGenes, a tool to probe tissue differentiation using a human fetal transcriptional atlas. Stem Cell Reports 4, 1112–1124 (2015).
Li, Y. et al. Identification of nephrotoxic compounds with embryonic stem-cell-derived human renal proximal tubular-like cells. Mol. Pharm. 11, 1982–1990 (2014).
Araoka, T. et al. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PloS ONE 9, e84881 (2014).
Toyohara, T. et al. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med. 4, 980–992 (2015).
Rabelink, T. J. & Little, M. H. Stromal cells in tissue homeostasis: balancing regeneration and fibrosis. Nat. Rev. Nephrol. 9, 747–753 (2013).
Imberti, B. et al. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci. Rep. 5, 8826 (2015).
Koulouridis, E. & Koulouridis, I. The loop of Henle as the milestone of mammalian kindey concentrating ability: a historical review. Acta Med. Hist. Adriat. 12, 413–428 (2014).
Jansen, J. et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 6, 26715 (2016).
Roessger, A., Denk, L. & Minuth, W. W. Potential of stem/progenitor cell cultures within polyester fleeces to regenerate renal tubules. Biomaterials 30, 3723–3732 (2009).
Song, J. J. et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 19, 646–651 (2013).
Bonandrini, B. et al. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng. Part A 20, 1486–1498, (2014).
Caralt, M. et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am. J. Transplant. 15, 64–75, (2015).
Little, M. H. Improving our resolution of kidney morphogenesis across time and space. Curr. Opin. Genet. Dev. 32, 135–143 (2015).
Acknowledgements
M.H.L. is a Senior Principal Research Fellow of the National Health and Medical Research Council (ID1042093). A.N.C. is an Australian Research Council (ARC) DECRA Postdoctoral Fellow (DE150100652). The laboratory is supported by funding from the NHMRC (ID1041277), NIH (DK107344-01) and the ARC (DP130102939).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to discussing the article's content, writing the article and reviewing or editing the article before submission.
Corresponding author
Ethics declarations
Competing interests
M.H.L. has previously (2014) received research funding from Organovo Inc.
Glossary
- Human embryonic stem cells
-
Pluripotent stem cells derived from the early preimplantation embryo; they are thought to arise from the epiblast.
- Induced pluripotent stem cell
-
Pluripotent stem cell generated from a somatic cell via transcriptional reprogramming. The approach was pioneered in mice in 2006 and can also be performed in human somatic cells.
- Pluripotent stem cells
-
Cells that have the potential to differentiate into any cell type of the body.
- Epiblast
-
One of two distinct layers of the inner cell mass of the preimplantation embryo. The epiblast can give rise to all three germ layers: ectoderm, mesoderm and endoderm; the kidneys are derived from the mesoderm.
- Primitive streak
-
Elongated region of cells along the axis of the embryo that represents the site of gastrulation. It arises via the movement of lateral cells toward the medial axis and gives rise to the endoderm and mesoderm of the embryo with the rostro-caudal and medial-lateral axes of the embryo defined by its position.
- Visceral endoderm
-
Extra-embryonic tissue that surrounds the epiblast before gastrulation.
- Node
-
Site where gastrulation occurs in the developing embryo.
- Paraxial mesoderm
-
Region of the trunk mesoderm that lies along the spinal cord and gives rise to bone, cartilage, skeletal muscle and dermis via somitogenesis. It is characterized by the expression of Tcf15, Tbx6 and Pax3.
- Lateral plate mesoderm
-
Region of the trunk mesoderm that is located most distally from the spinal cord and gives rise to the heart, smooth muscles, blood cells, endothelium, the spleen and limbs. It is characterized by the expression of Osr1, Foxf1 and Nkx2-5.
- Intermediate mesoderm
-
Region of the trunk mesoderm that develops between the paraxial mesoderm and the lateral plate mesoderm. It differentiates into the nephric duct and the nephrogenic mesenchyme, which give rise to the urogenital system including the kidney, the gonads, and the adrenal cortex. It is marked by the expression of Pax2, Lhx1 (anterior) and Hoxd11, Eya1 (posterior).
- Notochord
-
Midline structure along the axis of the embryo located ventrally to the neural tube that has a critical role in patterning during development.
- Nephrogenic cord
-
Non-epithelial mesodermal mass alongside the nephric duct that originates from the intermediary mesoderm and is marked by Osr1 and Wt1 expression. It gives rise to the mesonephric and metanephric mesenchyme that form the mesonephric tubules of the mesonephros and the nephrons of the kidney.
- Nephric duct
-
Epithelial tube structure derived from the intermediary mesoderm in both the pronephros and mesonephros that gives rise to the ureteric bud, which forms the collecting ducts and the ureter of the metanephros. It also contributes to the male reproductive tract but regresses in the female. Marked by Gata3, Lhx1, Pax2 and Ecad expression.
- Pronephros
-
First and most rostral excretory organ to form along the mammalian embryo axis. The pronephros degenerates as the mesonephros is formed.
- Mesonephros
-
Second excretory organ to form along the mammalian embryo axis. It is composed of mesonephric tubules and degenerates during fetal development with sexually dimorphic regression (some tubules are retained in males to form the rete testis).
- Metanephros
-
Final and permanent excretory organ to form in mammals. It arises as an interaction between the ureteric bud and the metanephric mesenchyme and its excretory function commences before birth.
- Anterior intermediate mesoderm
-
The most rostral portion of the forming intermediate mesoderm from which the nephric duct and the pronephric tubules arise. It is formed by the first cells to migrate from the primitive streak.
- Ureteric epithelium
-
Tissue derived from the ureteric bud that invades the metanephric mesenchyme and then branches dichotomously to form the ureteric tree.
- Posterior intermediate mesoderm
-
Most caudal portion of the forming intermediary mesoderm from which the nephrogenic mesenchyme arises. It is formed by cells that migrate out of the primitive streak at a later time point than cells that give rise to the anterior intermediate mesoderm.
- Metanephric mesenchyme
-
Caudal part of the nephrogenic mesenchyme that gives rise to the nephrons, the stromal interstitium and some vascular elements within the final metanephric kidney. Marked by the expression of Osr1, Six1, Six2, Eya1, Wt1 and Gdnf.
- Tailbud
-
This proliferating mass of cells at the caudal end of the embryo, sometimes referred to as the caudal cell mass or caudal eminence, is the source of cells that contribute to the elongating body axis.
- Ureteric bud
-
Epithelial bud that arises from the caudal nephric duct adjacent to the metanephric mesenchyme in response to GDNF, a chemoattractant secreted by the metanephric mesenchyme.
- Ureteric tree
-
Tree-like structure derived from the dichotomous branching of the ureteric epithelium that gives rise to the collecting ducts in the metanephros. The ends of each branch are called 'ureteric tips' and express Ret, Gfra1 and Wnt11.
- Cap mesenchyme
-
Derivative of the metanephric mesenchyme adjacent to the tips of the branching ureteric bud that gives rise to all of the cell types of the nephron via mesenchymal-to-epithelial transition; it is hence also referred to as the nephrogenic mesenchyme. It is marked by the expression of Osr1, Six2, Cited1, Eya1, Wt1, Pax2 and Gdnf.
- Organ-on-a-chip
-
Microfluidic cell culture chip that houses various cell types to mimic the 3D physiology (multicellular architecture, tissue-tissue interfaces, physicochemical and mechanical environment) of an organ.
- Artificial scaffolds
-
Structural element or framework used to hold cells or tissues together.
Rights and permissions
About this article
Cite this article
Little, M., Combes, A. & Takasato, M. Understanding kidney morphogenesis to guide renal tissue regeneration. Nat Rev Nephrol 12, 624–635 (2016). https://doi.org/10.1038/nrneph.2016.126
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.126
This article is cited by
-
Transforming growth factor beta signaling functions during mammalian kidney development
Pediatric Nephrology (2021)
-
Retinoic Acid Benefits Glomerular Organotypic Differentiation from Adult Renal Progenitor Cells In Vitro
Stem Cell Reviews and Reports (2021)
-
The FGF, TGFβ and WNT axis Modulate Self-renewal of Human SIX2+ Urine Derived Renal Progenitor Cells
Scientific Reports (2020)
-
Induction of human pluripotent stem cells into kidney tissues by synthetic mRNAs encoding transcription factors
Scientific Reports (2019)
-
Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation
Nature Communications (2019)