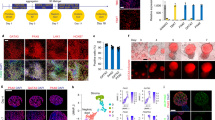
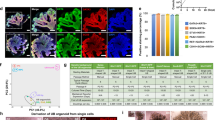
Abstract
Developing models of human kidney tissue in vitro is an important challenge in regenerative nephrology research, given the paucity of novel and effective therapies in kidney disease. However, the de novo generation of kidney tissues from human pluripotent stem cells (hPSCs) is challenging owing to the structural and functional complexity of the organ, as well its developmental origin from two distinct embryologic populations: the metanephric mesenchyme and the ureteric bud (UB). Directed differentiation strategies have been developed to generate kidney organoids containing nephron-like structures; we recently reported an efficient and practical method to generate UB tissues. Here, we describe a detailed step-by-step protocol for differentiation of hPSCs into three-dimensonal UB organoids that exhibit complex morphological development and the capacity to differentiate into functional collecting duct tissues. Over 3 d, hPSCs are induced into PAX2+GATA3+ pronephric (anterior) intermediate mesoderm fates in monolayer cultures at high efficiency. The cells are aggregated into three-dimensional spheroids, which then assemble and organize into nephric duct-like tissue over 4 d. When embedded into an extracellular matrix, the spheroids grow into UB organoids that recapitulate fetal branching morphogenesis for 1 week of culture. When switched to permissive conditions, the UB organoids spontaneously differentiate to form collecting duct principal cells. This approach provides robust and reproducible methods that can be readily adopted by users with basic experience in hPSC and organoid differentiation to generate UB tissues, which may be used to investigate human kidney development, model disease processes and catalyze further efforts in engineering functional kidney tissue.
Key points
-
Human pluripotent stem cells are differentiated into three-dimensional ureteric bud organoids that exhibit complex morphological development and the capacity to differentiate into functional collecting duct tissues.
-
The procedure accurately recapitulates early developmental stages and produces uretic bud structures showing branching via terminal bifurcation and stalk elongation. It can also be easily scaled as there is a high degree of inter- and intra-experimental consistency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ransick, A. et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev. Cell 51, 399–413 e397 (2019).
Park, J. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018).
England, A. R. et al. Identification and characterization of cellular heterogeneity within the developing renal interstitium. Development 147, dev190108 (2020).
Daniel, E. et al. Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21, 617–634 (2018).
Munro, D. A. D., Hohenstein, P. & Davies, J. A. Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci. Rep. 7, 3273 (2017).
Barak, H., Rosenfelder, L., Schultheiss, T. M. & Reshef, R. Cell fate specification along the anterior–posterior axis of the intermediate mesoderm. Dev. Dyn. 232, 901–914 (2005).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Costantini, F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip. Rev. Dev. Biol. 1, 693–713 (2012).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Tsujimoto, H. et al. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep. 31, 107476 (2020).
Howden, S. E. et al. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28, 671–684 e676 (2021).
Mae, S. I. et al. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem. Biophys. Res. Commun. 495, 954–961 (2018).
Mae, S. I. et al. Expansion of human iPSC-derived ureteric bud organoids with repeated branching potential. Cell Rep. 32, 107963 (2020).
Taguchi, A. & Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21, 730–746 e736 (2017).
Zeng, Z. et al. Generation of patterned kidney organoids that recapitulate the adult kidney collecting duct system from expandable ureteric bud progenitors. Nat. Commun. 12, 3641 (2021).
Shi, M. et al. Human ureteric bud organoids recapitulate branching morphogenesis and differentiate into functional collecting duct cell types. Nat. Biotechnol. 41, 252–261 (2023).
Sawada, K. & Aoyama, H. Fate maps of the primitive streak in chick and quail embryo: ingression timing of progenitor cells of each rostro-caudal axial level of somites. Int. J. Dev. Biol. 43, 809–815 (1999).
Parameswaran, M. & Tam, P. P. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev. Genet 17, 16–28 (1995).
Attia, L., Yelin, R. & Schultheiss, T. M. Analysis of nephric duct specification in the avian embryo. Development 139, 4143–4151 (2012).
Przepiorski, A. et al. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep. 11, 470–484 (2018).
Morizane, R. & Bonventre, J. V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc. 12, 195–207 (2017).
Sanchez-Ferras, O. et al. A coordinated progression of progenitor cell states initiates urinary tract development. Nat. Commun. 12, 2627 (2021).
Pepicelli, C. V., Kispert, A., Rowitch, D. H. & McMahon, A. P. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev. Biol. 192, 193–198 (1997).
Vega, Q. C., Worby, C. A., Lechner, M. S., Dixon, J. E. & Dressler, G. R. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc. Natl Acad. Sci. USA 93, 10657–10661 (1996).
Michos, O. et al. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 6, e1000809 (2010).
Qiao, J., Sakurai, H. & Nigam, S. K. Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc. Natl Acad. Sci. USA 96, 7330–7335 (1999).
Yuri, S., Nishikawa, M., Yanagawa, N., Jo, O. D. & Yanagawa, N. In vitro propagation and branching morphogenesis from single ureteric bud cells. Stem Cell Rep. 8, 401–416 (2017).
Toka, H. R., Toka, O., Hariri, A. & Nguyen, H. T. Congenital anomalies of kidney and urinary tract. Semin. Nephrol. 30, 374–386 (2010).
Kuure, S. & Sariola, H. Mouse models of congenital kidney anomalies. Adv. Exp. Med. Biol. 1236, 109–136 (2020).
Grobstein, C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science 118, 52–55 (1953).
Broda, T. R., McCracken, K. W. & Wells, J. M. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 14, 28–50 (2019).
McCracken, K. W., Howell, J. C., Wells, J. M. & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 (2011).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404 (2014).
Acknowledgements
This study was supported by funding from the National Institutes of Health National Institute for Diabetes and Digestive and Kidney Diseases and National Center for Advancing Translational Sciences (UH3 TR002155, R37 DK39773), and the Pediatric Center of Excellence at the University of Virginia (P50 DK096373). K.W.M. was supported by the National Institutes of Health training grant (T32 DK00772) and the Ben J. Lipps Fellowship from the American Society of Nephrology. M.S. was supported by National Natural Science Foundation of China (32100909) and Post-Doctor Research Project, West China Hospital, Sichuan University (2021HXBH016). Studies involving hPSCs were reviewed and approved by MGB Institutional Biosafety Committee (2011B000287). No new hESC lines were generated as part of this study.
Author information
Authors and Affiliations
Contributions
M.S., J.V.B. and K.W.M. conceived of this study and formulated experiments. M.S., J.V.B. and K.W.M. performed the experiments and wrote the manuscript. P.F. performed experiments and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.V.B. is an inventor on kidney organoid patents assigned to MGB, and is cofounder and holds equity in Goldfinch Bio. K.W.M. has kidney organoid patents pending. J.V.B.’s interests were reviewed and are managed by BWH and MGB in accordance with their conflict-of-interest policies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Melissa Little, Zhongwei Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Shi, M. et al. Nat. Biotechnol. 41, 252–261 (2023): https://doi.org/10.1038/s41587-022-01429-5
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, M., Fu, P., Bonventre, J.V. et al. Directed differentiation of ureteric bud and collecting duct organoids from human pluripotent stem cells. Nat Protoc 18, 2485–2508 (2023). https://doi.org/10.1038/s41596-023-00847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00847-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.