Abstract
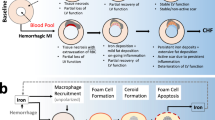
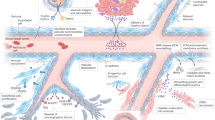
Despite efforts to restore tissue perfusion after myocardial infarction, coronary no-reflow—a failure to achieve adequate reperfusion of the cardiac microcirculation—is a common complication, which correlates with an increased incidence of death and disability. The treatment of ischaemic stroke is also plagued by no-reflow and, in the brain, a major cause of this phenomenon has been shown to be contractile microvascular pericytes irreversibly constricting capillaries and dying. We propose that cardiac pericytes, which are the second most-common cell type in the heart, impede reperfusion of coronary capillaries in a similar fashion to those in the brain after a stroke. Pericyte constriction might contribute to morbidity in patients by causing microvascular obstruction, even after successful treatment of coronary artery block. The similarity of the no-reflow phenomenon in the brain and in the heart suggests that cardiac pericytes are a novel therapeutic target for coronary no-reflow after myocardial infarction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
WHO. Cardiovascular diseases (CVDs) Fact Sheet No 317 [online], (2013).
British Heart Foundation. Coronary Heart Disease Statistics 2012 [online], (2012).
de Boer, M. J. et al. Limitation of infarct size and preservation of left ventricular function after primary coronary angioplasty compared with intravenous streptokinase in acute myocardial infarction. Circulation 90, 753–761 (1994).
Kawano, H. et al. Histopathological findings of the no-reflow phenomenon following coronary intervention for acute coronary syndrome. Int. Heart J. 46, 327–332 (2005).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Niccoli, G., Burzotta, F., Galiuto, L. & Crea, F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 54, 281–292 (2009).
Wu, K. C. et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97, 765–772 (1998).
Morishima, I. et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 36, 1202–1209 (2000).
Galiuto, L. et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J. Am. Coll. Cardiol. 51, 552–559 (2008).
Bolognese, L. et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109, 1121–1126 (2004).
Henriques, J. P. et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 107, 2115–2119 (2003).
Gibson, C. M. et al. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 105, 1909–1913 (2002).
Brosh, D. et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am. J. Cardiol. 99, 442–445 (2007).
Reffelmann, T. & Kloner, R. A. The “no-reflow” phenomenon: basic science and clinical correlates. Heart 87, 162–168 (2002).
Ames, A., Lewis Wright, R., Kowada, M., Thurston, J. M. & Majno, G. Cerebral ischemia. II. The no-reflow phenomenon. Am. J. Pathol. 52, 437–453 (1968).
Hauck, E. F., Apostel, S., Hoffmann, J. F., Heimann, A. & Kempski, O. Capillary flow and diameter changes during reperfusion after global cerebral ischemia studied by intravital video microscopy. J. Cereb. Blood Flow Metab. 24, 383–391 (2004).
Krug, A., de Rochemont, W. M. & Korb, G. Blood supply of the myocardium after temporary coronary occlusion. Circ. Res. 19, 57–62 (1966).
Kloner, R. A., Ganote, C. E. & Jennings, R. B. The 'no-reflow' phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 54, 1496–1508 (1974).
Przyklenk, K. & Kloner, R. A. 'Reperfusion injury' by oxygen-derived free radicals? Effect of superoxide dismutase plus catalase, given at the time of reperfusion, on myocardial infarct size, contractile function, coronary microvasculature, and regional myocardial blood flow. Circ. Res. 64, 86–96 (1989).
Engler, R. L., Schmid-Schönbein, G. W. & Pavelec, R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am. J. Pathol. 111, 98–111 (1983).
de la Torre, J. C., Fortin, T., Saunders, J. K., Butler, K. & Richard, M. T. The no-reflow phenomenon is a post-mortem artifact. Acta Neurochir. 115, 37–42 (1992).
Ørn, S. et al. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur. Heart J. 30, 1978–1985 (2009).
Beek, A. M., Nijveldt, R. & van Rossum, A. C. Intramyocardial hemorrhage and microvascular obstruction after primary percutaneous coronary intervention. Int. J. Cardiovasc. Imaging 26, 49–55 (2010).
Wu, K. C. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J. Cardiovasc. Magn. Reson. 14, 68 (2012).
Cheseboro, J. H. et al. Thrombolysis in Myocardial Infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase: clinical findings through hospital discharge. Circulation 76, 142–154 (1987).
White, H. D. & Braunwald, E. Applying the open artery theory: use of predictive survival markers. Eur. Heart J. 19, 1132–1139 (1998).
Ito, H. et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 93, 1993–1999 (1996).
Bolognese, L., Falsini, G., Liistro, F., Angioloi, P. & Ducci, K. Epicardial and microvascular reperfusion with primary percutaneous coronary intervention. Ital. Heart J. 6, 447–452 (2005).
Heusch, G. et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation 120, 1822–1836 (2009).
Leineweber, K. et al. Intense vasoconstriction in response to aspirate from stented saphenous vein aortocoronary bypass grafts. J. Am. Coll. Cardiol. 47, 981–986 (2006).
Kleinbongard, P. et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ. Res. 108, 344–352 (2011).
Jaffe, R., Charron, T., Puley, G., Dick, A. & Strauss, B. H. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117, 3152–3156 (2008).
Porto I. et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur. Heart J. 33, 2928–2938 (2012).
Dalkara, T. & Arsava, E. M. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J. Cereb. Blood Flow Metab. 32, 2091–2099 (2012).
Yan, A. T. et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 114, 32–39 (2006).
Schwartz, B. G. & Kloner, R. A. Coronary no reflow. J. Mol. Cell. Cardiol. 52, 873–882 (2012).
Heusch, G. et al. The coronary circulation in cardioprotection: more than just one confounder. Cardiovasc. Res. 94, 237–245 (2012).
Peppiatt, C. M., Howarth, C., Mobbs, P. & Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704 (2006).
Yemisci, M. et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 15, 1031–1037 (2009).
Hamilton, N. B., Attwell, D. & Hall, C. N. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front. Neuroenergetics 2, 5 (2010).
Crawford, C. et al. Extracellular nucleotides affect pericyte-mediated regulation of rat in situ vasa recta diameter. Acta Physiol. (Oxf.) 202, 241–251 (2011).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Puro, D. G. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation 14, 1–10 (2007).
Lacar, B., Herman, P., Platel, J. C., Kubera, C., Hyder, F. & Bordey, A. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J. Neurosci. 32, 16435–16448 (2010).
Fernández-Klett, F., Offenhauser, N., Dirnagl, U., Priller, J. & Lindauer, U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl Acad. Sci. USA 107, 22290–22295 (2010).
Lovick, T. A., Brown, L. A. & Kay, B. J. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience 92, 47–60 (1999).
Borysova, L., Wray, S., Eisner, D. A. & Burdyga, T. How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium 54, 163–174 (2013).
Nees, S. et al. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am. J. Physiol. Heart Circ. Physiol. 302, H69–H84 (2012).
O'Farrell, F., Coleman, E., Kendrick, S. & Attwell, D. Microanatomy of pericytes in the rat ventricular myocardium. Proc. Physiol. Soc. 27, C84 (2012).
Tilton, R. G., Kilo, C. & Williamson, J. R. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc. Res. 18, 325–335 (1979).
Chintalgattu, V. et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci. Transl. Med. 5, 187ra69. (2013).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Armulik, A. et al. C. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Fernández-Klett, F. et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow Metab. 33, 2091–2099 (2013).
Jespersen, S. N. & Østergaard, L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J. Cereb. Blood Flow Metab. 32, 264–277 (2012).
Juchem, G. et al. Pericytes in the macrovascular intima: possible physiological and pathogenetic impact. Am. J. Physiol. Heart Circ. Physiol. 298, H754–H770 (2010).
Nees, S., Weiss, D. R. & Juchem, G. Focus on cardiac pericytes. Pflügers Arch. 465, 779–787 (2013).
Yamagishi, S.-I. et al. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 290, 973–978 (2002).
Crea, F., Camici, P. G. & Bairey Merz, C. N. Coronary microvascular dysfunction: an update. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/eht513.
Lee, S. et al. Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nat. Comm. 3, 1054–1058 (2012).
Mitchell, T. S., Bradley, J., Robinson, G. S., Shima, D. T. & Ng Y.-S. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 11, 141–151 (2008).
Niccoli, G., Spaziani, C. & Crea, F. Left ventricular remodeling and 1-year clinical follow-up of the REOPEN-AMI trial. J. Am. Coll. Cardiol. 63, 1454–1455 (2014).
Matsugi, T., Chen, Q. & Anderson, D. R. Adenosine-induced relaxation of cultured bovine retinal pericytes. Invest. Ophthalmol. Vis. Sci. 38, 2695–701 (1997).
Ramachandran, E., Frank, R. N. & Kennedy, A. Effects of endothelin on cultured bovine retinal microvascular pericytes. Invest. Ophthalmol. Vis. Sci. 34, 586–595 (1993).
Niccoli, G. et al. Endothelin-1 and acute myocardial infarction: a no-reflow mediator after successful percutaneous myocardial revascularization. Eur. Heart J. 29, 1843–1850 (2006).
Galiuto, L. et al. Ischemia-reperfusion injury at the microvascular level: treatment by endothelin A-selective antagonist and evaluation by myocardial contrast echocardiography. Circulation 102, 3111–3116 (2000).
Sakagami, K, Wu, D. M. & Puro, D. G. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J. Physiol. 521, 637–650 (1999).
Taniyama, Y. et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 30, 1193–1199 (1997).
Dore-Duffy, P., Katychev, A., Wang, X. & Van Buren, E. CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow Metab. 26, 613–624 (2006).
Chen, C. W. et al. Human pericytes for ischemic heart repair. Stem Cells 31, 305–316 (2013).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Campagnolo, P. et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation 121, 1735–1745 (2010).
Katare, R. et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 109, 894–906 (2011).
Watanabe, N. et al. Three-dimensional microstructural abnormality of the coronary capillary network after myocardial reperfusion—comparison between 'reflow' and 'no-reflow'. Circ. J. 68, 868–872 (2004).
Ebert, C. J. Handbuch der Lehre von den Geweben des Menschen und der Tiere, Vol. 1 (Leipzig, 1871).
Rouget, C. Memoire sur le developpement, la structures et les proprietes des capillaires sanguins et lymphatiques [French]. Arch. Physiol. Norm. Pathol. 5, 603–633 (1873).
Armulik, A., Genové, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Chan-Ling, T. et al. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am. J. Pathol. 165, 1301–1313 (2004).
Göritz, C. et al. A pericyte origin of spinal cord scar tissue. Science 333, 238–242 (2011).
Acknowledgements
We thank the Fondation Leducq, Wellcome Trust, European Research Council, Medical Research Council (UK), and Rosetrees Trust for funding. We thank P. C. Adams (Newcastle Hospitals NHS Foundation Trust, UK), I. S. Cohen (SUNY Stony Brook University, NY, USA), R. Jolivet (University College London, UK), and A. Mishra (University College London, UK) for comments on the manuscript. We thank A. Nishiyama (University of Connecticut, CT, USA) and D. Dietrich (University Clinic Bonn, Germany) for providing NG2–DsRed mice.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
O'Farrell, F., Attwell, D. A role for pericytes in coronary no-reflow. Nat Rev Cardiol 11, 427–432 (2014). https://doi.org/10.1038/nrcardio.2014.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2014.58
This article is cited by
-
PBX/Knotted 1 homeobox-2 (PKNOX2) is a novel regulator of myocardial fibrosis
Signal Transduction and Targeted Therapy (2024)
-
In vivo methods for imaging blood–brain barrier function and dysfunction
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Single-cell transcriptional profile of ACE2 in healthy and failing human hearts
Science China Life Sciences (2021)
-
Microvascular bioengineering: a focus on pericytes
Journal of Biological Engineering (2019)
-
Bone marrow pericyte dysfunction in individuals with type 2 diabetes
Diabetologia (2019)