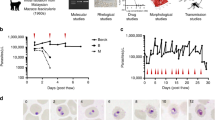
Abstract
The tightly controlled induction of Plasmodium falciparum gametocytes in large-scale culture is a fundamental requirement for malaria drug discovery applications including, but not limited to, high-throughput screening. This protocol uses magnetic separation for isolation of hemozoin-containing parasites in order to (i) increase parasitemia, (ii) decrease hematocrit and (iii) introduce higher levels of young red blood cells in a culture simultaneously within 2–4 h. These parameters, along with red blood cell lysis products that are generated through schizont rupture, are highly relevant for enabling optimum induction of gametocytogenesis in vitro. No other previously published protocols have applied this particular approach for parasite isolation and maximization of fresh red blood cells before inducing gametocytogenesis, which is essential for obtaining highly synchronous gametocyte classical stages on a large scale. In summary, 500–1,000 million stage IV gametocytes can be obtained within 16 d from an initial 10 ml of asexual blood-stage culture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sinden, R.E., Carter, R., Drakeley, C. & Leroy, D. The biology of sexual development of Plasmodium: the design and implementation of transmission-blocking strategies. Malar. J. 11, 70 (2012).
Baker, D.A. Malaria gametocytogenesis. Mol. Biochem. Parasitol. 172, 57–65 (2010).
Adjalley, S.H. et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. USA 108, E1214–E1223 (2011).
Lucantoni, L. et al. Identification of MMV malaria box inhibitors of Plasmodium falciparum early-stage gametocytes, using a luciferase-based high-throughput assay. Antimicrob. Agents Chemother. 57, 6050–6062 (2013).
Duffy, S. & Avery, V.M. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 12, 408 (2013).
Duez, J. et al. Splenic retention of Plasmodium falciparum gametocytes to block the transmission of malaria. Antimicrob. Agents Chemother. 59, 4206–4214 (2015).
Lucanoni, L. et al. A simple and predictive phenotypic high content imaging assay for Plasmodium falciparum mature gametocytes to identify malaria transmission blocking compounds. Sci. Rep. 5, 16414 (2015).
Lelievre, J. et al. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence 'transmission blocking' assay. PloS One 7, e35019 (2012).
Peatey, C.L., Spicer, T.P., Hodder, P.S., Trenholme, K.R. & Gardiner, D.L. A high-throughput assay for the identification of drugs against late-stage Plasmodium falciparum gametocytes. Mol. Biochem. Parasitol. 180, 127–131 (2011).
D'Alessandra, S. et al. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J. Antimicrob. Chemother. 68, 2048–2058 (2013).
Tanaka, T.Q. & Williamson, K.C. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol. Biochem. Parasitol. 177, 160–163 (2011).
Tanaka, T.Q. et al. A quantitative high throughput assay for identifying gametocytocidal compounds. Mol. Biochem. Parasitol. 188, 20–25 (2013).
Bowman, J.D. et al. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the Malaria Box. Antimicrob. Agents Chemother. 58, 811–819 (2014).
Wang, Z. et al. A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PloS One 9, e93825 (2014).
Cevenini, L. et al. Multicolor bioluminescence boosts malaria research: quantitative dual-color assay and single-cell imaging in Plasmodium falciparum parasites. Anal. Chem. 86, 8814–8821 (2014).
Tao, D. et al. Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol. Cell Proteomics 13, 2705–2724 (2014).
Silvestrini, F. et al. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 143, 100–110 (2005).
Creek, D.J. & Barrett, M.P. Determination of antiprotozoal drug mechanisms by metabolomics approaches. Parasitology 141, 83–92 (2014).
Tibúrcio, M. et al. Early gametocytes of the malaria parasite Plasmodium falciparum specifically remodel the adhesive properties of infected erythrocyte surface. Cell Microbiol. 15, 647–659 (2013).
Ruecker, A. et al. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob. Agents Chemother. 58, 7292–7302 (2014).
Miguel-Blanco, C. et al. Imaging-based HTS assay to identify new molecules with transmission-blocking potential against P. falciparum female gamete formation. Antimicrob. Agents Chemother. 59, 3298–3305 (2015).
Miura, K. et al. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PloS One 8, e57909 (2013).
Roncalés, M., Vidal-Mas, J., Leroy, D. & Herreros, E. Comparison and optimization of different methods for the in vitro production of Plasmodium falciparum gametocytes. J. Parasitol. Res. 2012, 927148 (2012).
Ponnudurai, T., Meuwissen, J.H., Leeuwenberg, A.D., Verhave, J.P. & Lensen, A.H. The production of mature gametocytes of Plasmodium falciparum in continuous cultures of different isolates infective to mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 76, 242–250 (1982).
Dyer, M. & Day, K.P. Regulation of the rate of asexual growth and commitment to sexual development by diffusible factors from in vitro cultures of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 68, 403–409 (2003).
Williams, J.L. Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am. J. Trop. Med. Hyg. 60, 7–13 (1999).
Trager, W. & Gill, G.S. Enhanced gametocyte formation in young erythrocytes by Plasmodium falciparum in vitro. J. Protozool. 39, 429–432 (1992).
Trager, W., Gill, G.S., Lawrence, C. & Nagel, R.L. Plasmodium falciparum: enhanced gametocyte formation in vitro in reticulocyte-rich blood. Exp. Parasitol. 91, 115–118 (1999).
Carter, R. & Miller, L.H. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Org. 57, 37–52 (1979).
Bruce, M.C., Alano, P., Duthie, S. & Carter, R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 2, 191–200 (1990).
Akompong, T., Eksi, S., Williamson, K. & Haldar, K. Gametocytocidal activity and synergistic interactions of riboflavin with standard antimalarial drugs against growth of Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 44, 3107–3111 (2000).
Fivelman, Q.L. et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154, 119–123 (2007).
Cranmer, S.L., Magowan, C., Liang, J., Coppel, R.L. & Cooke, B.M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91, 363–365 (1997).
Reader, J. et al. Nowhere to hide: interrogating different metabolic parameters of Plasmodium falciparum gametocytes in a transmission blocking drug discovery pipeline towards malaria elimination. Malar. J. 14, 213 (2015).
Frankland, S. et al. Serum lipoproteins promote efficient presentation of the malaria virulence protein PfEMP1 at the erythrocyte surface. Eukaryot. Cell 6, 1584–1594 (2007).
Kafsack, B.F. et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507, 248–252 (2014).
Ke, H. et al. The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J. Biol. Chem. 289, 34827–34837 (2014).
MacRae, J.I. et al. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 11, 67 (2013).
Hliscs, M. et al. Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell. Microbiol. 17, 207–225 (2015).
Tibúrcio, M. et al. Specific expression and export of the Plasmodium falciparum Gametocyte EXported Protein-5 marks the gametocyte ring stage. Malar. J. 14, 334 (2015).
Cabrera, M. & Cui, L. In vitro activities of primaquine-schizonticide combinations on asexual blood stages and gametocytes of Plasmodium falciparum. Antimicrob. Agents Chemother. 59, 7650–7656 (2015).
Carter, R., Ranford-Cartwright, L.C. & Alano, P. In Protocols in Molecular Parasitology (Totowa, New Jersey: Humana Press Inc., 1993).
Brancucci, N.M.B., Goldowitz, I., Buchholz, K., Werling, K. & Marti, M. An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nat. Protoc. 10, 1131–1142 (2015).
Eksi, S., Suri, A. & Williamson, K.C. Sex- and stage-specific reporter gene expression in Plasmodium falciparum. Mol. Biochem. Parasitol. 160, 148–151 (2008).
Nilsen, A. et al. Quinolone-3-Diarylethers: a new class of drugs for a new era of malaria eradication. Sci. Transl. Med. 20, 5 (2013).
Phillips, M.A. et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl. Med. 7, 7 (2015).
Trager, W. & Jensen, J.B. Human malaria parasites in continuous culture. Science 193, 673–675 (1976).
Lambros, C. & Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418–420 (1979).
Duffy, S. & Avery, V.M. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am. J. Trop. Med. Hyg. 86, 84–92 (2012).
Zhang, J., Chung, T.D.Y. & Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4, 67–73 (1999).
Acknowledgements
The authors thank and acknowledge the Australian Red Cross Blood Bank for the provision of fresh red blood cells, without which this research could not have been performed. We thank Medicines for Malaria Venture (MMV) for the supply of the MMV Malaria Box. This work was supported by the Australian Research Council (LP120200557 to V.M.A.) and MMV.
Author information
Authors and Affiliations
Contributions
S.D. conceived, developed and performed the majority of the experimental research involved in optimizing this protocol. S.D. contributed significantly to troubleshooting and undertook the studies using the product of the protocol. S.D. wrote the manuscript and revisions. J.P.H. contributed to writing of the first draft and revision of the final manuscript, and assisted with troubleshooting the protocol. S.L. contributed to the final manuscript, provided technical assistance during the development and optimization of the induction protocol and formatted images. S.L. also designed Figure 2. V.M.A. supervised the drug discovery program, provided guidance and contributed to the interpretation of data and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Duffy, S., Loganathan, S., Holleran, J. et al. Large-scale production of Plasmodium falciparum gametocytes for malaria drug discovery. Nat Protoc 11, 976–992 (2016). https://doi.org/10.1038/nprot.2016.056
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.056
This article is cited by
-
Safe drugs with high potential to block malaria transmission revealed by a spleen-mimetic screening
Nature Communications (2023)
-
Development and optimizing a simple and cost-effective medium for in vitro culture of Plasmodium berghei-ANKA strain with conserving its infectivity in BALB/c mice
BMC Research Notes (2022)
-
Protocols for Plasmodium gametocyte production in vitro: an integrative review and analysis
Parasites & Vectors (2022)
-
CRISPR/Cas9-engineered inducible gametocyte producer lines as a valuable tool for Plasmodium falciparum malaria transmission research
Nature Communications (2021)
-
Investigation of factors affecting the production of P. falciparum gametocytes in an Indian isolate
3 Biotech (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.