Abstract
The neurobiology of schizophrenia (SZ) may be altered in older versus younger adults with SZ, as less frequent episodes of symptom exacerbation and increased sensitivity to medications are observed in older age. The goal of this study was to examine the effect of age and diagnosis on glutamate and cerebral blood flow (rCBF) in adults with SZ and healthy controls. Young and older adults with SZ and healthy controls were recruited to participate in this study. Participants completed a neuropsychological battery and neuroimaging that included optimized magnetic resonance spectroscopy to measure anterior cingulate (AC) glutamate (Glu) and glutamine (Gln) and arterial spin labeling evaluation for rCBF. Regression analyses revealed significant effects of age with Glu, Gln, Gln/Glu, and AC white matter (WM) rCBF. Glu and WM rCBF decreased linearly with age while Gln and Gln/Glu increased linearly with age. Glu was lower in adults with SZ compared with healthy controls and in older adults versus younger adults but there was no interaction. Glu and WM rCBF were correlated with the UCSD Performance-Based Skills Assessment (UPSA) and processing speed, and the correlations were stronger in the SZ group. In the largest sample to date, lower Glu and elevated Gln/Glu levels were observed in adults with SZ and in older subjects. Contrary to expectation, these results do not show evidence of accelerated Glu aging in the anterior cingulate region in SZ compared with healthy controls.
Similar content being viewed by others
INTRODUCTION
The neurobiology of schizophrenia (SZ) may be altered in older versus younger adults with SZ since older adults with SZ have less frequent episodes of symptom exacerbation and increased sensitivity to medications (Jeste and Maglione, 2013). Post-mortem studies of several brain regions including the anterior cingulate have shown altered regional morphologies due to aging (Torii et al, 2012) and glutamatergic receptor expression (Zavitsanou et al, 2002). However, there have been very few studies that examine the effects of aging in schizophrenia using advanced magnetic resonance techniques at 3 T. Glutamate (Glu), the primary excitatory neurotransmitter in the human brain, has been implicated in the pathophysiology of SZ since Glu N-methyl-D-aspartate receptor (NMDAR) antagonists such as ketamine and phencyclidine (PCP) induce positive, negative, and cognitive symptoms similar to SZ (Krystal et al, 1994). Glu can be non-invasively measured in vivo using 1H-MRS, and this technique has been utilized in numerous studies to investigate Glu in the medial frontal lobe, including the anterior cingulate (AC), in various stages of the illness: prodrome, first episode, and chronic (Bustillo et al, 2010; Egerton et al, 2012; Natsubori et al, 2014; Rowland et al, 2013; Tayoshi et al, 2009; Theberge et al, 2002, 2003). A recent meta-analysis showed a decrease in Glu that was potentiated with age as measured with 1H-MRS in the medial frontal lobe of adults with SZ (Marsman et al, 2013).
In conjunction with 1H-MRS, utilization of arterial spin labeling (ASL) to quantify regional cerebral blood flow (rCBF) may provide additional insight into the pathophysiology of the illness. Blood flow is tightly coupled to metabolism and neural activity in the steady state brain (Busija et al, 2007), so that lower rCBF is taken as a marker of diminished neural activity. Previous ASL studies of schizophrenia have shown a relationship between rCBF and positive and negative symptoms as well as processing speed (Horn et al, 2009; Pinkham et al, 2011; Wright et al, 2015), and highlighted a reduction in white matter (WM) perfusion with aging (Wright et al, 2014). Thus, these two techniques are complementary in that 1H-MRS provides information about glutamatergic tissue levels and ASL captures rCBF that is modulated by local glutamatergic neurotransmission.
The goals of this study were to examine the effect of age and diagnosis in the largest sample to date on AC Glu and AC rCBF and to examine the relationship between AC Glu and AC rCBF. Glutamine (Gln) is the major metabolite of Glu, and it can also be detected reliably using a specifically optimized MRS technique for detection of these metabolites (Bustillo et al, 2015; Wijtenburg et al, 2013). In addition, Gln/Glu ratio is thought by some to reflect glutamatergic neurotransmission via glutamate–glutamine (Bustillo et al, 2010; Shirayama et al, 2010).
Because the spectroscopic voxel contained both gray matter (GM) and white matter (WM), we examined the relationship between Glu and both AC GM rCBF and AC WM rCBF. Single-voxel MRS cannot differentiate between gray and white matter glutamatergic metabolites. Therefore, both GM and WM rCBF were examined. Historically, GM has been linked to Glu neurotransmission; however, emerging evidence highlights the importance of Glu neurotransmission in WM (Kukley et al, 2007) and that significant white matter alterations have been observed in SZ (Dias, 2012; Kochunov et al, 2014). Overall, we hypothesized that Glu and rCBF would be reduced as a function of age and diagnosis such that older adults with SZ (OSZ) would have the lowest Glu and rCBF levels compared with younger adults with SZ (YSZ), younger healthy adults (YHC), and older healthy adults (OHC).
MATERIALS AND METHODS
Participant Characteristics
This study was approved by the University of Maryland Baltimore Institutional Review Board, and all participants provided informed consent prior to study enrollment. Adults with SZ were evaluated for capacity to consent to ensure that each participant understood the study and its procedures. A total of 188 participants were enrolled in this study: 48 YSZ, 47 OSZ, 54 OHC, and 39 OHC. Age group assignment was determined by median split with the median being 36 years old such that younger adults were of ages 18–36 and older adults were of ages 37–62. There were 167 participants with MRS data and 105 participants with ASL data. Thirty-six of the 167 MRS datasets overlap with a previous study by Rowland et al (Rowland et al, 2016), and 30 of the 105 ASL datasets overlap with two previous studies by Wright et al (Wright et al, 2014, 2015). Participant demographics are detailed in Table 1, and a breakdown of the antipsychotic medications being taking by adults with SZ are summarized in Supplementary Table 1. Each patient with SZ was characterized and evaluated with the Structured Clinical Interview for DSM-IV (SCID), the Brief Psychiatric Rating Scale (BPRS), and Brief Negative Symptom Scale (BNSS). Inclusion criteria for adults with schizophrenia and healthy controls were (1) no current or past neurological condition, head trauma, or focal findings on MR scan, (2) no substance abuse in past 6 months or lifetime dependence excluding nicotine, and (3) no contraindication for MRI scanning. Healthy controls were included in this study if they did not have a past or current Axis I DSM-IV diagnosis and had no family history of a psychotic disorder. Participants were administered the UCSD Performance–Based Skills Assessment (UPSA) (Patterson et al, 2001) to assess functional capacity. Since processing speed and working memory deficits are among the most robust cognitive impairments in schizophrenia (Knowles et al, 2010; Lee and Park, 2005), participants were assessed with the Digit-Symbol Coding subtest from the Wechsler Adult Intelligence Scale (WAIS) for processing speed and the Digit-Span Sequencing task from WAIS for verbal working memory (Wechsler, 1997).
Imaging Parameters
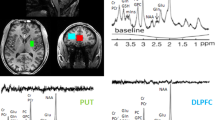
Participants were scanned using a Siemens TIM Trio 3 T MR system with a 32-channel phased array head coil located at the University of Maryland Center for Brain Imaging Research. MP-RAGE images were prescribed parallel with the AC-PC line. The spectroscopic voxel was placed in the medial prefrontal region along the midline (Figure 1a). Spectroscopy data were acquired using phase rotation STEAM (PR-STEAM) (Bustillo et al, 2015; Wijtenburg et al, 2013; Wijtenburg and Knight-Scott, 2011): TR/TM/TE=2000/10/6.5-ms, VOI~24-cm3 (R-L: 30 mm x A-P: 40mm x F-H: 20mm and fixed across subjects), NEX=128, 2.5-kHz spectral width, 2048 complex points, and phases: ϕ1=135°, ϕ2=22.5°, ϕ13=112.5°, ϕADC=0° optimized for detection of Glu (Wijtenburg and Knight-Scott, 2011) and shown to have good reproducibility for Glu and glutamine, separately (Bustillo et al, 2015; Wijtenburg et al, 2013). A water reference (NEX=16) was also acquired for phase and eddy current correction as well as quantification. Spectroscopy post-processing methods can be found in the supplement. Metabolite levels were reported in institutional units, and all metabolites except Gln with percent standard deviation Cramer Rao Lower Bounds (CRLBs)⩽20% were included in statistical analyses. For Gln, metabolites with CRLB⩽25% were included in statistical analyses, which are consistent with published reports (Bustillo et al, 2014; Shirayama et al, 2010). For measurement of rCBF, a pseudo-continuous arterial spin labeling (pCASL) sequence was applied with the following parameters: TR/TE=4000/16 ms, FOV=220 × 220 mm, Number of slices=23, Slice thickness=5 mm, Voxel size=3.4 × 3.4 × 5.0 mm3, Bandwidth=1594 Hz/pixel, 136 measurements, labeling offset=90 mm, labeling duration of 1.85 s, and post labeling delay of 0.93 s (Wu et al, 2007). Arterial spin labeling post-processing methods can be found in the supplement.
(a) T1-weighted images showing voxel placement in the medial prefrontal lobe. (b) Representative spectra (gray, top) with corresponding LCModel fit (red) and residue (gray, bottom) shown above. Examination of the fit residues shows noise with no definitive peaks, showing excellent fits to the data. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Statistical Analyses
Demographic, clinical, and cognitive variables were analyzed in SPSS version 23.0 using chi-square for categorical variables and regression models for continuous variables with significance set at p<0.05. To examine the effects of age and diagnosis, a linear regression model incorporating age, diagnosis, and the interaction term was applied to main outcome variables: glutamatergic metabolites (Glu, Gln, and Gln/Glu) and CBF measures (AC GM rCBF, cingulated WM rCBF, and whole brain (WB) CBF). If the regression model was not significant, then each term was evaluated for its significance and the regression model was adjusted accordingly. Age was centered by subtracting out the median age of 36 and then divided by 10 to compute a change in dependent variable per decade in age. Centering the data to the median age shifted interpretation of the regression coefficient for diagnosis to the estimated difference between HC and SZ at the overall median age rather than the difference at age 0. Age divided by 10 shifts the interpretation of our estimates from ‘change per year’ to ‘change per 10 years,’ which seems helpful in a cohort whose ages range over 4 decades. Similar exploratory models for the other MRS metabolites were applied as well. Unstandardized coefficients (β)±SE, t, degrees of freedom (df), and p-values were reported for regression models. Bonferroni correction for multiple comparisons was set to p<0.0083 (0.05/6) for the linear regression analyses. Exploratory regression analyses were conducted for the other six metabolites found in the spectrum with p<0.05/6, and results are summarized in the Supplement.
Pearson product moment correlations were conducted between main outcome variables: glutamatergic metabolites and CBF values. Next, correlation analyses were conducted between glutamatergic metabolites and CBF values with cognitive measures across combined groups and clinical symptoms in the adults with SZ group. If the overall correlations were significant, further correlation analyses were performed on each diagnostic group. Fisher’s Z transformation was used to test the significance of the difference in magnitude of these correlations. Benjamini–Hochberg false discovery rate at p<0.05 was used for correction of multiple correlation analyses between glutamatergic metabolites, CBF metrics, cognitive measures, and clinical symptom assessments. This correction was applied across Table 2 and Supplementary Tables 3 and 4 that shows the uncorrected p-values with significance set at p<0.008 after FDR correction.
RESULTS
Participant Characteristics
Participant demographics, clinical measures, and cognitive measures are summarized in Table 1. Regression results are summarized in the Supplement. Using covariates of smoking status and CPZ had no effect on the study results (Supplementary Information).
Glutamatergic Metabolites
A representative spectrum and corresponding LCModel fit is shown in Figure 1b, highlighting the excellent spectral quality acquired in this study. Mean line width (LW) and signal-to-noise ratio (SNR) as reported by LCModel and mean GM, WM, and CSF proportions are summarized in the Supplementary Table 2. Mean metabolite levels are summarized in Table 2. There were significant effects for GM, WM, and CSF between the groups similar to another study (Bustillo et al, 2014); however, these differences in voxel composition are accounted for with partial volume correction applied to the metabolite concentrations as discussed in the Method section. MRS data were not included in further analyses with LCModel reported SNR of <10 or LW >0.1. One of the 167 MRS data sets was excluded due to poor SNR and broad LW. There were no Glu data sets excluded based on CRLB⩽20%, and there were 16 out of 166 Gln datasets excluded due to CRLB>25%.
For Glu, examination of regression analyses incorporating age, diagnosis, and the interaction term revealed a significant linear relationship with significant age (βage=−0.39±0.047, t=−8.23,df=165,p<0.001) and diagnosis effects (βdx=−0.44±0.13, t=−3.47, df=165, p=0.001) (Figure 2a). Thus, Glu levels, reported in institutional units, change -0.39 per 10 year increase in age and the average distance between HC and SZ Glu levels is -0.44, suggesting that SZ have lower Glu levels. With CRLB⩽25%, regression models for age, diagnosis, and interaction revealed a significant linear relationship between age and Gln (βage=0.082±0.03, t=2.71, df=149, p=0.007) (Figure 2b). Thus, Gln levels increase 0.082 per 10 year increase in age, and the average distance between HC and SZ Gln levels is negligible. With CRLB⩽20% for Gln, the significant finding of a linear relationship between age and Gln held (Supplementary Information).
Neuroimaging measures correlated with age by group. Age was centered (median age of 36) and divided by 10 in order to obtain results in reference to changes in measures per decade of age. (a) Plots of Glu levels as a function age in both SZ (▴) and HC (•). The significant linear model with age and diagnosis as main effects revealed lower Glu in SZ than HC and a decrease in Glu levels with aging in both groups. (b) Plots of Gln levels as a function age in both SZ (▴) and HC (•). A linear regression model with age and diagnosis effects revealed that age was the only significant predictor of Gln levels such that Gln increased with age. There was not a significant diagnosis effect as evidenced by similar increasing slope for both groups. (c) Plots of Gln/Glu ratio levels as a function age in both SZ (▴) and HC (•). The significant linear regression model with age and diagnosis as main effects revealed that Gln/Glu is elevated in SZ and increases with aging in both groups. (d) Plots of AC rCBF levels as a function age in both SZ (▴) and HC (•). Application of a linear regression model revealed that rCBF is lower in older adults versus younger adults (as evidenced by the negative slope).
For Gln/Glu and age, a linear regression model revealed a significant relationship with age (βage=0.021±0.004, t=5.79, df=149, p<0.001) and diagnosis (βdx=0.026±0.01, t=2.72, df=149, p=0.007) effects (Figure 2c). Thus, Gln/Glu levels increase 0.021 per 10 years of life, and the average distance between HC and SZ shows that SZ have higher levels of Gln/Glu than HC. There were no significant interaction effects in any of the three significant linear models for Glu, Gln, and Gln/Glu so the term was removed.
rCBF
Mean rCBF levels are summarized in Table 2. Since the spectroscopic voxel contained both GM (mean across groups ~52%) and WM (mean across groups ~36%), rCBF analyses were conducted for AC GM rCBF and WM rCBF as well as WB CBF, resulting in a total of 105 participants with ASL data. For AC GM rCBF, linear regression analyses with age, diagnosis, and interaction revealed no significant effects (p=0.20). Linear regression model analyses between age and WM rCBF revealed a significant linear relationship (p=0.001) with age (βage=−1.18±0.34, t=−3.46, df=104, p=0.001) and not diagnosis (p=0.09; Figure 2d). Thus, WM rCBF decreases every 10 years of age. Linear regression model analyses with age and diagnosis as the main effects for WB CBF were not significant (p=0.071). Exploratory analysis of the CBF from the MRS voxel produced similar results (Supplementary Information).
Correlations between Glutamatergic Metabolites and rCBF
Correlation results for glutamatergic metabolites and CBF are summarized in Table 3. Glu was not significantly correlated with AC GM rCBF or WB CBF. Glu and AC WM rCBF were significantly correlated across combined groups (r=0.42, df=103, p<0.001; Supplementary Figure 1). When broken down according to diagnosis, there was a significant correlation in SZ (r=0.49, df=51, p<0.001) and not in HC (r=0.24, df=50, p=0.089), and the difference in magnitude between the two correlations was not significant (Z-score=1.45, p=0.15). Further examination into the SZ data revealed a significant relationship between Glu and AC WM rCBF in YSZ (r=0.52, df=24, p=0.006) compared with OSZ (r=0.38, df=25, p=0.052); however, the correlation coefficients were not significantly different (Z-score=0.60, p=0.55). Thus, higher Glu was associated with higher AC WM rCBF, and direction of the relationship was similar in HC and SZ. Due to the CRLB cut-off of 25% or less, the number of participants with glutamatergic and CBF data decreased to 94 for Gln and Gln/Glu measurements. There were no significant correlations between Gln and any CBF metrics. For Gln/Glu, there was a significant finding between Gln/Glu and AC WM rCBF (r=−0.32, df=92, p=0.002) across groups (Supplementary Figure 2). The correlation coefficient in HC (r=−0.38, df=46, p=0.008) was significant at trend level compared with the SZ group (r=−0.26, df=44, p=0.079), and the difference in correlation coefficients was not significant (Z-score=−0.63, p=0.53). Thus, as Gln/Glu decreases, AC WM rCBF increases and this relationship is similar in both diagnostic and age groups.
Correlations with Cognitive Measures
Correlation results for cognitive measures with glutamatergic metabolites and rCBF levels are summarized in Supplementary Table 3. Across both groups, Glu was moderately significantly correlated with UPSA total score (r=0.31, df=139, p<0.001), processing speed (r=0.39, df=158, p<0.001), and verbal working memory (r=0.23, df=155, p=0.005). Separating by diagnostic groups showed a positive relationship between Glu and UPSA in SZ (r=0.27, df=73, p=0.018) compared with a negative relationship between Glu and UPSA in HC (r=−0.12, df=64, p=0.34), and the Z-score showed that the correlations were significantly different (Z-score=−2.31, p=0.021). For processing speed, the strong positive correlation between Glu and processing speed was present in HC (r=0.28, df=78, p=0.011) and SZ (r=0.36, df=78, p=0.001) with the correlations in each group being at trend level (HC) or significant (SZ), but the difference in magnitude of the correlation was not (Z-score=−0.51, p=0.61). Further examination into age groups revealed that the relationship between processing speed and Glu was more pronounced in OSZ (r=0.35, df=38, p=0.026) compared with YSZ (r=0.20, df=40, p=0.22). In terms of diagnostic groups, Glu and verbal working memory correlations were not significant in either HC or SZ.
Across both groups, Gln was correlated with UPSA (r=−0.25, df=124, p=0.006) and processing speed (r=−0.23, df=142, p=0.005), but not verbal working memory. When separated by diagnostic group, the correlations between UPSA and Gln were very similar and not significant in HC (r=−0.23) and in SZ (r=−0.21) with the Z-score revealing no significant difference (Z-score=0.12, p=0.91). The relationship between Gln and processing speed was similar in HC (r=−0.15) versus SZ (r=−0.24) and not significant for either correlation or in terms of the Z-score (Z-score=0.53, p=0.60).
For Gln/Glu, there was a significant relationship between Gln/Glu and processing speed (r=−0.39, df=142, p<0.001) and UPSA (r=−0.37, df=124, p<0.001), but not with verbal working memory across groups. In SZ, there was a significant correlation between Gln/Glu and processing speed (r=−0.37, df=67, p=0.002), and a similar relationship was observed in HC (r=−0.27, df=73, p=0.018). When further delineated by age group within the SZ group, OSZ had a trend level negative correlation between processing speed and Gln/Glu (r=−0.42, df=33, p=0.012) while YSZ had a non-significant correlation (r=−0.22). Overall, even though the correlation coefficients between processing speed and Gln/Glu in HC versus SZ (Z-score=0.66, p=0.51) and YSZ versus OSZ (Z-score=0.89, p=0.37) were not significant, there was still a strong negative relationship across groups such that lower processing speed scores were associated with higher Gln/Glu levels. In terms of UPSA, the SZ group had a more negative correlation at trend level (r=−0.32, df=62, p=0.01) compared with HC (r=−0.15, df=60, p=0.24), but the Z-score between correlations was not significant (Z-score=−0.99, p=0.32).
In terms of CBF measures, there were no significant correlations between AC GM rCBF with any cognitive measures across all groups. AC WM rCBF was significantly associated with UPSA (r=0.29, df=93, p=0.005) and at trend level with processing speed (r=0.26, df=101, p=0.008), but not verbal working memory with both diagnostic groups combined. When separated by diagnostic group, UPSA and AC WM rCBF were positively correlated in SZ (r=0.36, df=48, p=0.011) at trend level, and negatively correlated in HC (r=−0.12, df=43, p=0.046), and the two correlations were significantly different between groups (Z-score=−2.28, p=0.023). Since processing speed and AC WM rCBF were significant at trend level (p=0.008), separation by diagnostic group revealed a significant moderate correlation in SZ (r=0.37, df=50, p=0.002) but not in HC (r=0.002, df=49, p=0.99). The difference in magnitude between the correlations was not significant (Z-score=−1.91, p=0.056). When further delineated by age group, while not significant, the association between processing speed and Glu was more pronounced in YSZ (r=0.42, df=24, p=0.033) compared with OSZ (r=0.22, df=24, p=0.28). In terms of WB CBF, there was no association with UPSA, verbal working memory, or processing speed.
Correlations with Clinical Symptoms
Supplementary Table 4 summarizes the correlations between clinical variables, glutamatergic metabolites, and CBF. In SZ, there were no significant correlations between BNSS, BPRS total, BPRS positive, and BPRS negative scores and Glu (p>0.36), Gln (p>0.07), Gln/Glu (p>0.16), AC GM rCBF (p>0.24), WM rCBF (p>0.53), and WB rCBF (p>0.17).
DISCUSSION
This is the largest study to examine glutamatergic and regional cerebral blood flow differences between adults with SZ and healthy controls. This study used a 1H-MRS technique specifically tailored to detect Glu, and an optimal ASL technique to measure rCBF (Alsop et al, 2014; Wijtenburg and Knight-Scott, 2011). The major findings showed adults with SZ had lower Glu than HC and lower Glu and AC WM rCBF levels in older adults versus younger adults irrespective of diagnosis. The inverse was true in terms of Gln/Glu with it increasing with age in both groups and it was higher in SZ compared with HC. Gln levels increased with age regardless of diagnosis. Contrary to expectation (Marsman et al, 2013), these results do not show evidence of accelerated Glu or Gln/Glu aging in the anterior cingulate region in SZ compared with healthy controls. These data also suggest that adults with SZ do not experience accelerated aging in AC rCBF.
Few studies have examined the effects of age on Glu in SZ. One study observed that chronic SZ participants had the lowest Glx compared with ultra high risk and first episode participants in the medial prefrontal cortex (Natsubori et al, 2014), while another study found reduced Glx in the anterior cingulate and white matter centrum semiovale in SZ but no age effect (Rowland et al, 2013). Bustillo et al (2014) did not find age or diagnosis effects for anterior cingulate Glu but did find alterations in Gln and Gln/Glu in SZ (Bustillo et al, 2014). The reduction in Glu levels in SZ observed in this study may be potentially explained by a combination of abnormal Glu receptor function and reduced neuropil (Dickstein et al, 2007; Rubio et al, 2012). Overall, this study provides further evidence of a reduction in Glu with SZ similar to other studies (Natsubori et al, 2012, 2003) and with age (Hadel et al, 2013).
Glutamine and Gln/Glu were elevated as a function of age and Gln/Glu was higher in schizophrenia. These results are consistent with studies of healthy aging showing Gln increases with age in humans and rodents (Hadel et al, 2013; Harris et al, 2014). With respect to schizophrenia, higher AC Gln was observed in antipsychotic naïve first episode patients and reduced in chronic medicated SZ (Theberge et al, 2002, 2003). In contrast, two other studies did not observe any AC Gln differences between medicated patients and controls (Tayoshi et al, 2009; Theberge et al, 2003). In a large study, Gln and Gln/Glu were elevated in participants with SZ (combination of first episode and chronic patients), and increased with aging across groups. These results are also consistent with a study reporting increased Gln/Glu with PCP, an NMDAR antagonist, in rodents (Iltis et al, 2009). Overall, lower Glu and higher Gln/Glu in schizophrenia indicates altered glutamatergic system possibly due to NMDAR hypofunction, but the exact mechanism remains to be determined.
Application of ASL to the study of cerebral blood flow alterations in SZ is relatively new. Previous studies in SZ utilized different acquisition and quantification methods for measuring rCBF; however, none of these studies examined SZ and age (Horn et al, 2009; Kindler et al, 2013; Ota et al, 2014; Pinkham et al, 2011; Scheef et al, 2010; Zhu et al, 2015). Two studies showed decreased GM rCBF in the anterior cingulate and frontal regions in SZ versus controls (Kindler et al, 2013; Zhu et al, 2015) but two other studies reporting no differences in medial frontal regions (Ota et al, 2014; Pinkham et al, 2011), similar to our results. In terms of WM rCBF, one did not observe any differences in SZ while another observed that WM rCBF decreased with age and faster in SZ (Pinkham et al, 2011; Wright et al, 2014). Similarly, this study found an aging effect of AC WM rCBF, which is also consistent with work showing rCBF decrease across life span (Lu et al, 2011).
In this study, a correlation between Glu and WM rCBF, and not AC GM rCBF, was observed. Examination of the tissue components within the voxel showed averages of 53% GM, 35% WM, and 12% CSF. We expected Glu to correlate with GM rCBF as Glu concentrations are higher in GM compared with WM (Michaelis et al, 1993). However, Glu in WM is highly detectable with MRS, and several studies highlight the important role of Glu neurotransmission in WM including signal propagation (Kukley et al, 2007). Also, this relationship was more pronounced in SZ, which converges nicely with reports of significant white matter alterations in SZ (Dias, 2012; Kochunov et al, 2014). We are unable to determine whether Glu in WM or GM is driving the correlation observed here. Future studies incorporating advanced MR techniques like short TE echo planar imaging EPSI (Ebel and Maudsley, 2003) are needed to differentiate white and gray matter Glu levels.
The potential impact of antipsychotic medication is inherent to the majority of schizophrenia research. The effect of antipsychotic medication on MRS measures remains unknown. Some off medication studies reported higher levels of glutamatergic metabolites (de la Fuente-Sandoval et al, 2011, 2013a, 2013b, 2015; Kegeles et al, 2012; Kraguljac et al, 2013), whereas others reported reduced or no difference (Goto et al, 2012; Wood et al, 2008). However, higher levels of glutamatergic metabolites do not appear to be specific to the off medication state since several studies reported higher glutamatergic metabolites in medicated patients (Aoyama et al, 2011; Bustillo et al, 2014; Chang et al, 2007; Theberge et al, 2003) and in treatment resistant patients taking medication (Demjaha et al, 2014; Egerton et al, 2012; Mouchlianitis et al, 2016). Finally, a rodent MRS study showed no effect of 6 months antipsychotic treatment on Glu or Gln levels (Bustillo et al, 2006). Hence, the concept that glutamatergic metabolites are elevated in schizophrenia only in the off medication state and decline or normalize with antipsychotic treatment is not fully supported by empirical evidence.
Study limitations include voxel size and number, medication, macromolecules, and cross-sectional study. The voxel size translates to measures of Glu and rCBF from multiple regions within the frontal lobe and precludes conclusions about specific regions, as does the use of one voxel. Medications are a potential confound but statistical control of CPZ units indicated no effect. A limitation to utilizing CPZ units is that they only control for potency of dopamine D2 receptors. Nevertheless, studies are needed to determine the effects of medication on glutamatergic metabolites and rCBF in across the illness duration. Macromolecules are present in very short TE spectra. Similar to many other studies, the LCModel basis set of macromolecules was used for fitting the spectra. There are no studies that examined macromolecule levels between patients and controls. If differences exist, using the same MM basis set to fit both patients and controls could possibly impact the results. Studies comparing macromolecule levels in schizophrenia and control samples are needed. This study was cross-sectional and thus no conclusions can be made regarding the causal mechanisms underlying the reported observations; longitudinal studies are needed to examine the changes of Glu and rCBF with aging in SZ.
Overall, this is the first large scale study to show decreased Glu, increased Gln/Glu, and decreased WM rCBF in SZ from the anterior cingulate, suggesting an altered neurobiological state as determined by neurochemical and physiological metrics. These data do not support the notion that Glu declines more rapidly as function of age in SZ compared with controls; rather, these two groups have similar trajectories for Glu, Gln/Glu, and WM rCBF in this region.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L et al (2014). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73: 102–116.
Aoyama N, Theberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RW et al (2011). Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry 198: 448–456.
Busija DW, Bari F, Domoki F, Louis T (2007). Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev 56: 89–100.
Bustillo J, Barrow R, Paz R, Tang J, Seraji-Bozorgzad N, Moore GJ et al (2006). Long-term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology 31: 751–756.
Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C et al (2014). Increased Glutamine in Patients Undergoing Long-term Treatment for Schizophrenia: A Proton Magnetic Resonance Spectroscopy Study at 3 T. JAMA Psychiatry 71: 265–272.
Bustillo JR, Rediske N, Jones T, Rowland LM, Abbott C, Wijtenburg SA (2015). Reproducibility of phase rotation stimulated echo acquisition mode at 3T in schizophrenia: Emphasis on glutamine. Magn Reson Med 75: 498–502.
Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C et al (2010). 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry 15: 629–636.
Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K (2007). Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry 62: 1396–1404.
de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A (2013a). Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol 16: 471–475.
de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L et al (2013b). Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70: 1057–1066.
de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J et al (2011). Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 36: 1781–1791.
de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Solis-Vivanco R et al (2015). Cortico-Striatal GABAergic and Glutamatergic Dysregulations in Subjects at Ultra-High Risk for Psychosis Investigated with Proton Magnetic Resonance Spectroscopy. Int J Neuropsychopharmacol 19: 3.
Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM et al (2014). Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry 75: e11–e13.
Dias AM (2012). The Integration of the Glutamatergic and the White Matter Hypotheses of Schizophrenia's Etiology. Curr Neuropharmacol 10: 2–11.
Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR (2007). Changes in the structural complexity of the aged brain. Aging Cell 6: 275–284.
Ebel A, Maudsley AA (2003). Improved spectral quality for 3D MR spectroscopic imaging using a high spatial resolution acquisition strategy. Magn Reson Imag 21: 113–120.
Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK et al (2012). Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology 37: 2515–2521.
Goto N, Yoshimura R, Kakeda S, Nishimura J, Moriya J, Hayashi K et al (2012). Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat 8: 119–122.
Hadel S, Wirth C, Rapp M, Gallinat J, Schubert F (2013). Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Reson Imag 38: 1480–1487.
Harris JL, Yeh HW, Swerdlow RH, Choi IY, Lee P, Brooks WM (2014). High-field proton magnetic resonance spectroscopy reveals metabolic effects of normal brain aging. Neurobiol Aging 35: 1686–1694.
Horn H, Federspiel A, Wirth M, Muller TJ, Wiest R, Wang JJ et al (2009). Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry 194: 130–138.
Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J et al (2009). Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR Biomed 22: 737–744.
Jeste DV, Maglione JE (2013). Treating older adults with schizophrenia: challenges and opportunities. Schizophr Bull 39: 966–968.
Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X et al (2012). Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69: 449–459.
Kindler J, Jann K, Homan P, Hauf M, Walther S, Strik W et al (2013). Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull 41: 163–170.
Knowles EE, David AS, Reichenberg A (2010). Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry 167: 828–835.
Kochunov P, Chiappelli J, Wright SN, Rowland LM, Patel B, Wijtenburg SA et al (2014). Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry Res 223: 148–156.
Kraguljac NV, White DM, Reid MA, Lahti AC (2013). Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70: 1294–1302.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214.
Kukley M, Capetillo-Zarate E, Dietrich D (2007). Vesicular glutamate release from axons in white matter. Nat Neurosci 2007: 311–320.
Lee J, Park S (2005). Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol 114: 599–611.
Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B et al (2011). Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 21: 1426–1434.
Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE (2013). Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull 39: 120–129.
Michaelis T, Merboldt KD, Bruhn H, Hanicke W, Frahm J (1993). Absolute concentrations fo metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology 187: 219–227.
Mouchlianitis E, Bloomfield MA, Law V, Beck K, Selvaraj S, Rasquinha N et al (2016). Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull 42: 744–752.
Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y et al (2014). Reduced frontal glutamate+glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull 40: 1128–1139.
Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H et al (2014). Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res 154: 113–118.
Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV (2001). UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 27: 235–245.
Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R (2011). Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res 194: 64–72.
Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA et al (2013). In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull 39: 1096–1104.
Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N et al (2016). Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry 73: 166–174.
Rubio MD, Drummond JB, Meador-Woodruff JH (2012). Glutamate receptor abnormalities in schizophrenia: implications for innovative treatments. Biomol Ther (Seoul) 20: 1–18.
Scheef L, Manka C, Daamen M, Kuhn KU, Maier W, Schild HH et al (2010). Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology 256: 253–260.
Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E et al (2010). Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage 49: 2783–2790.
Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J et al (2009). Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS). Schizophr Res 108: 69–77.
Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N et al (2003). Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry 160: 2231–2233.
Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J et al (2002). Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 159: 1944–1946.
Torii Y, Iritani S, Sekiguchi H, Habuchi C, Hagikura M, Arai T et al (2012). Effects of aging on the morphologies of Heschl's gyrus and the superior temporal gyrus in schizophrenia: a postmortem study. Schizophr Res 134: 137–142.
Wechsler D (1997). Adult Intelligence Scale. Psychological Coporation: San Antonio, TX, USA.
Wijtenburg SA, Gaston FE, Spieker EA, Korenic SA, Kochunov P, Hong LE et al (2013). Reproducibility of phase rotation STEAM at 3 T: Focus on glutathione. Magn Reson Med 72: 603–609.
Wijtenburg SA, Knight-Scott J (2011). Very short echo time improves the precision of glutamate detection at 3 T in 1H magnetic resonance spectroscopy. J Magn Reson Imaging 34: 645–652.
Wood SJ, Berger GE, Wellard RM, Proffitt T, McConchie M, Velakoulis D et al (2008). A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naive and early-treated first episode psychosis. Schizophr Res 102: 163–170.
Wright SN, Hong LE, Winkler AM, Chiappelli J, Nugent K, Muellerklein F et al (2015). Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum Brain Mapp 36: 3793–3804.
Wright SN, Kochunov P, Chiappelli J, McMahon RP, Muellerklein F, Wijtenburg SA et al (2014). Accelerated white matter aging in schizophrenia: role of white matter blood perfusion. Neurobiol Aging 35: 2411–2418.
Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J (2007). A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 58: 1020–1027.
Zavitsanou K, Ward PB, Huang XF (2002). Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology 27: 826–833.
Zhu J, Zhuo C, Qin W, Xu Y, Xu L, Liu X et al (2015). Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res 63: 28–35.
Acknowledgements
All authors report no biomedical financial interests or potential conflicts of interest. The authors would like to acknowledge support from NIH to R01MH094520 (LMR), T32MH067533 (SAW), and R01DA027680 (LEH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Wijtenburg, S., Wright, S., Korenic, S. et al. Altered Glutamate and Regional Cerebral Blood Flow Levels in Schizophrenia: A 1H-MRS and pCASL study. Neuropsychopharmacol 42, 562–571 (2017). https://doi.org/10.1038/npp.2016.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.172
This article is cited by
-
Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of 1H-magnetic resonance spectroscopy studies
Molecular Psychiatry (2022)
-
A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia
Molecular Psychiatry (2021)
-
Magnetic Resonance Spectroscopy in Schizophrenia: Evidence for Glutamatergic Dysfunction and Impaired Energy Metabolism
Neurochemical Research (2019)
-
Salivary kynurenic acid response to psychological stress: inverse relationship to cortical glutamate in schizophrenia
Neuropsychopharmacology (2018)
-
Glutamate imaging (GluCEST) reveals lower brain GluCEST contrast in patients on the psychosis spectrum
Molecular Psychiatry (2017)