Abstract
The Hippo pathway is an important regulator of organ size and tumorigenesis. It is unclear, however, how Hippo signalling provides the cellular building blocks required for rapid growth. Here, we demonstrate that transgenic zebrafish expressing an activated form of the Hippo pathway effector Yap1 (also known as YAP) develop enlarged livers and are prone to liver tumour formation. Transcriptomic and metabolomic profiling identify that Yap1 reprograms glutamine metabolism. Yap1 directly enhances glutamine synthetase (glul) expression and activity, elevating steady-state levels of glutamine and enhancing the relative isotopic enrichment of nitrogen during de novo purine and pyrimidine biosynthesis. Genetic or pharmacological inhibition of GLUL diminishes the isotopic enrichment of nitrogen into nucleotides, suppressing hepatomegaly and the growth of liver cancer cells. Consequently, Yap-driven liver growth is susceptible to nucleotide inhibition. Together, our findings demonstrate that Yap1 integrates the anabolic demands of tissue growth during development and tumorigenesis by reprogramming nitrogen metabolism to stimulate nucleotide biosynthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Piccolo, S., Dupont, S. & Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 (2014).
Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Hensley, C. T., Wasti, A. T. & DeBerardinis, R. J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013).
Dibble, C. C. & Manning, B. D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 15, 555–564 (2013).
Her, G. M., Chiang, C. C., Chen, W. Y. & Wu, J. L. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio). FEBS Lett. 538, 125–133 (2003).
Spitsbergen, J. M. et al. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol. Pathol. 28, 705–715 (2000).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Benhamouche, S. et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev. Cell 10, 759–770 (2006).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Yuan, M., Breitkopf, S. B., Yang, X. & Asara, J. M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7, 872–881 (2012).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Schmidt, A. et al. Differential expression of glutamine synthetase and cytochrome P450 isoforms in human hepatoblastoma. Toxicology 281, 7–14 (2011).
Bioulac-Sage, P. et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 46, 740–748 (2007).
Nault, J. C. et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 60, 1983–1992 (2014).
Li, H. et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 32, 38–47 (2012).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Lian, I. et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 24, 1106–1118 (2010).
Rosenbluh, J. et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473 (2012).
Tao, J. et al. Activation of β-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology 147, 690–701 (2014).
Varelas, X. et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev. Cell 18, 579–591 (2010).
Barry, E. R. et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493, 106–110 (2013).
Yuneva, M. O. et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 15, 157–170 (2012).
Kung, H. N., Marks, J. R. & Chi, J. T. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 7, e1002229 (2011).
van der Vos, K. E. et al. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat. Cell Biol. 14, 829–837 (2012).
Howell, J. J., Ricoult, S. J., Ben-Sahra, I. & Manning, B. D. A growing role for mTOR in promoting anabolic metabolism. Biochem. Soc. Trans. 41, 906–912 (2013).
Mayers, J. R. & Vander Heiden, M. G. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 40, 130–140 (2015).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Cunningham, J. T., Moreno, M. V., Lodi, A., Ronen, S. M. & Ruggero, D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157, 1088–1103 (2014).
Bott, A. J. et al. Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metab. 22, 1068–1077 (2015).
Tardito, S. et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 17, 1556–1568 (2015).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Mayers, J. R. et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 20, 1193–1198 (2014).
Vander Heiden, M. G. Targeting cancer metabolism: a therapeutic window opens. Nat. Rev. Drug Discov. 10, 671–684 (2011).
Tumaneng, K. et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 14, 1322–1329 (2012).
Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013).
Robitaille, A. M. et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 339, 1320–1323 (2013).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Miller, E. et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 19, 955–962 (2012).
Sorrentino, G. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366 (2014).
Wang, Z. et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl Acad. Sci. USA 111, E89–98 (2014).
Adler, J. J. et al. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc. Natl Acad. Sci. USA 110, 17368–17373 (2013).
Adler, J. J. et al. Amot130 adapts atrophin-1 interacting protein 4 to inhibit yes-associated protein signaling and cell growth. J. Biol. Chem. 288, 15181–15193 (2013).
Anakk, S. et al. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 5, 1060–1069 (2013).
DeRan, M. et al. Energy stress regulates Hippo-YAP signaling involving AMPK-mediated regulation of Angiomotin-like 1 protein. Cell Rep. 9, 495–503 (2014).
Wang, W. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 (2015).
Mo, J. S. et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 17, 500–510 (2015).
Enzo, E. et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 34, 1349–1370 (2015).
Park, Y. Y. et al. Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology 63, 159–172 (2016).
Hansen, C. G., Ng, Y. L., Lam, W. L., Plouffe, S. W. & Guan, K. L. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 25, 1299–1313 (2015).
Her, G. M., Yeh, Y. H. & Wu, J. L. 435-bp liver regulatory sequence in the liver fatty acid binding protein (L-FABP) gene is sufficient to modulate liver regional expression in transgenic zebrafish. Dev. Dyn. 227, 347–356 (2003).
Thermes, V. et al. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech. Dev. 118, 91–98 (2002).
Kurita, R. et al. Suppression of lens growth by αA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev. Biol. 255, 113–127 (2003).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Goessling, W. et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev. Biol. 320, 161–174 (2008).
Collins, J. E., White, S., Searle, S. M. & Stemple, D. L. Incorporating RNA-seq data into the zebrafish Ensembl genebuild. Genome Res. 22, 2067–2078 (2012).
Luo, W., Friedman, M. S., Shedden, K., Hankenson, K. D. & Woolf, P. J. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10, 161 (2009).
Galli, G. G. et al. Prdm5 regulates collagen gene transcription by association with RNA polymerase II in developing bone. PLoS Genet. 8, e1002711 (2012).
Sudol, M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 9, 2145–2152 (1994).
Gaffney, C. J. et al. Identification, basic characterization and evolutionary analysis of differentially spliced mRNA isoforms of human YAP1 gene. Gene 509, 215–222 (2012).
Xia, J., Sinelnikov, I. V., Han, B. & Wishart, D. S. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res. 43, W251–257 (2015).
Deuel, T. F., Louie, M. & Lerner, A. Glutamine synthetase from rat liver. Purification, properties, and preparation of specific antisera. J. Biol. Chem. 253, 6111–6118 (1978).
Bucking, C., Lemoine, C. M. & Walsh, P. J. Waste nitrogen metabolism and excretion in zebrafish embryos: effects of light, ammonia, and nicotinamide. J. Exp. Zool. A 319, 391–403 (2013).
Skaper, S. D., O’Brien, W. E. & Schafer, I. A. The influence of ammonia on purine and pyrimidine nucleotide biosynthesis in rat liver and brain in vitro. Biochem. J. 172, 457–464 (1978).
Morin, N., Vallaeys, T., Hendrickx, L., Natalie, L. & Wilmotte, A. An efficient DNA isolation protocol for filamentous cyanobacteria of the genus Arthrospira. J. Microbiol. Methods 80, 148–154 (2010).
Acknowledgements
This work was supported by an Irwin Arias Postdoctoral Fellowship (A.G.C.) and Liver Scholar Award (A.G.C.) from the American Liver Foundation, an HDDC Pilot Feasibility Grant from the Harvard Digestive Disease Center, P30 DK034854 (A.G.C., D.Y.), NIH NIGMS T32GM007753 (K.L.H.), NIH NCI 5K08CA172288 (K.J.E.), NIH NIDDK R01DK60322 (D.Y.R.S.), NIH NIDDK R01DK090311 (W.G.), NIH K08DK105351 (D.Y.), R01AR064036 (F.D.C.) and R01DK099559 (F.D.C.), and the Packard Foundation (D.Y.R.S.). J.M.A. is partially supported by NIH NCI 5P01CA120964 and 5P30CA006516. G.G.G. is supported by an American-Italian Cancer Foundation postdoctoral research fellowship. K.J.E. was a Robert Black Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-109-10). D.Y. is a Gilead Sciences Scholar in Liver Disease. W.G. is supported by the Claudia Adams Barr Program for Innovative Cancer Research, and is a Pew Scholar in the Biomedical Sciences.
Author information
Authors and Affiliations
Contributions
A.G.C. and W.G. conceived the study, reviewed results and wrote the manuscript. K.L.H. contributed to experimental design. A.G.C. and K.L.H. performed the majority of the experiments and data analysis. K.K.B. performed cell culture experiments and immunoblotting. K.J.E. and D.Y.R.S. generated lf:Yap fish and K.J.E. performed pathological analysis of liver tumours. S.B., K.O’C., A.T. and S.N. assisted in zebrafish experiments. M.Y., E.C.L. and J.M.A. developed methods and analysed metabolomics samples. G.G.G. performed ChIP experiments. S.C. and Y.H. analysed RNA-seq data sets. D.Y., A.M., D.E.C., F.D.C., J.M.A., Y.H. and D.Y.R.S. provided overall input. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Hepatocyte-specific overexpression of activated Yap causes hepatomegaly and accelerates DMBA-induced liver tumor formation.
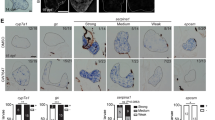
(a) Schematic diagram of the construct used to generate transgenic fish with hepatocyte-specific (fapb10a) expression of yap1S87A, abbreviated to lf:Yap. (b) Liver histology (H&E stain) in transverse sections of WT and lf:Yap larvae at 5 dpf. Scale bars: 50 μm (upper) and 20 μm (lower) for zoomed images. (c) Quantification of differentiated hepatocyte frequency in WT and lf:Yap larvae at 10 dpf, as determined by FACS. n = 5 biologically independent WT and lf:Yap single cell suspensions, each of which was derived from 10 larvae; see Supplementary Table 4. ∗∗P < 0.01, two-sided Student’s t-test, values represent the mean ± s.e.m. (d) Time course of hepatomegaly during early adulthood as determined by quantification of fluorescent liver area. n = 10, 6, 6, 4, 10, 8, 6 and 4 WT 3 wpf, WT 8 wpf, WT 12 wpf, WT 16 wpf, lf:Yap3 wpf, lf:Yap 8 wpf, lf:Yap 12 wpf and lf:Yap 16 wpf zebrafish respectively; see Supplementary Table 4. ∗P < 0.05, two-sided Student’s t-test, values represent the mean ± s.e.m. (e) Histological assessment of transverse sections from liver of WT and lf:Yap adults. H&E staining at low (10x) and high magnification (25x). Periodic-acid Schiff (PAS) stain for hepatic glycogen (pink inclusions). Bile duct morphogenesis determined by 2F11 staining. Cell proliferation as determined by PCNA staining. Scale bars: 200 μm and 50 μm for zoomed images. (f) Tumor heterogeneity in lf:Yap transgenics exposed to DMBA. Tumors include hepatocellular carcinoma (HCC), HCC with sarcomatoid features, HCC with peliosis/spongiosis-hepatis-like change, and cholangiocarcinoma (CCA). Scale bars: 2 mm for dissected images, 200 μm for histology and 50 μm for zoomed images. (g) Table illustrating the incidence of fibrosis, as determined by Sirius Red staining, glycogen, sarcomatoid cytology, peliosis/spongiosis-hepatis-like change and ascites in DMBA-induced liver tumors. n = 4 and 19 WT and lf:Yap liver tumors respectively.
Supplementary Figure 2 Yap alters expression of metabolism-related genes and enhances glul expression.
(a) Gene set enrichment (GSEA) derived from RNAseq of WT and lf:Yap adult livers identifies a conserved Yap target gene signature. (b) Gene ontology (GO) analysis of the top 20 biological processes upregulated by Yap. Biological processes related to metabolism are highlighted in red. (c) RNAseq analysis of the Wnt target genes cyclind1, axin2, cd44 in dissected WT and lf:Yap livers. n = 3 WT and lf:Yap adult livers; see Supplementary Table 4. Values represent the mean ± s.e.m. (d) qPCR validation of Wnt target genes axin2 and cyclind1 in adult zebrafish livers. n = 3 WT and lf:Yap adult livers; see Supplementary Table 4. Values represent the mean ± s.e.m. (e) RNAseq analysis of yap and taz expression in dissected WT and lf:Yap livers. n = 3 WT and lf:Yap adult livers; see Supplementary Table 4. ∗∗P < 0.01, two-sided Student’s t-test, values represent the mean ± s.e.m.
Supplementary Figure 3 Yap transcriptionally upregulates GLUL in an evolutionarily conserved fashion.
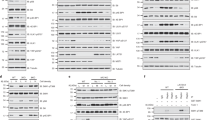
(a) ChIP qPCR analysis of transcriptional activity (H3K27ac) at the glula promoter in dissected WT and lf:Yap livers. Shown is the average of 2 biologically independent WT and lf:Yap adult liver chromatin preps, each of which was derived from a pooled sample of 2; see Supplementary Table 4. (b) Luciferase GLUL reporter assay using truncated promoter constructs in Hek293 cells expressing GFP, YAP or YAP1S127A. n = 3 biologically independent replicates; see Supplementary Table 4. Values represent the mean ± s.e.m. (c) ChIP qPCR analysis of YAP and TEAD4 enrichment at the GLUL promoter in Hep3B and HepG2 cells. Shown is the average of 2 biologically independent replicates; see Supplementary Table 4.
Supplementary Figure 4 Yap reprograms nitrogen metabolism by enhancing GLUL-dependent anabolic assimilation of ammonia for de novo nucleotide biosynthesis.
(a) Immunohistochemical detection of GLUL in WT, lf:Yap transgenic livers and DMBA-induced lf:Yap liver tumors. Scale bar, 50 μm. (b) Ammonia excretion rates in individual WT and lf:Yap adult fish. n = 16 and 12 WT and lf:Yap adult zebrafish respectively; see Supplementary Table 4. Values represent the mean ± s.e.m. (c) Steady-state abundance of urea in WT and lf:Yap livers as determined by selected reaction monitoring (SRM) analysis. n = 5 WT and lf:Yap adult livers; see Supplementary Table 4. Values represent the mean ± s.e.m. (d) Abundance of 15N-labelled Guanosine (M + 2 fraction) from methanol extracted WT and lf:Yap liver lysates, as determined by LC-MS/MS via SRM. n = 5 biologically independent WT and lf:Yap adult liver lysates; see Supplementary Table 4. ∗P < 0.05, two-sided Student’s t-test, values represent the mean ± s.e.m. (e) Abundance of 15N-labelled Cytosine (M + 2 fraction) from methanol extracted WT and lf:Yap liver lysates, as determined by LC-MS/MS via SRM. n = 5 biologically independent WT and lf:Yap adult liver lysates; see Supplementary Table 4. Values represent the mean ± s.e.m. (f) Percentage of 15N-labelled Glutamine isotopologues in WT and lf:Yap transgenic liver lysates following ammonia assimilation in the presence or absence or MSO. n = 5 biologically independent WT and lf:Yap adult liver lysates; see Supplementary Table 4. Values represent the mean ± s.e.m. (g) Percentage of 15N-labelled Histidine isotopologues in WT and lf:Yap transgenic liver lysates following ammonia assimilation in the presence or absence or MSO. n = 5 biologically independent WT and lf:Yap adult liver lysates; see Supplementary Table 4. Values represent the mean ± s.e.m.
Supplementary Figure 5 GLUL activity and nucleotide biosynthesis contribute to Yap-induced hepatomegaly and the growth of liver cancer cells.
(a) RT-PCR validation of morpholinos targeting splice sites in glula and glulb, resulting in alternative transcripts (indicated by arrow heads). (b) Glul activity in WT and lf:Yap larval extracts derived from larvae exposed to MSO from 3–5 dpf. n = 8, 7, 9 and 9 biologically independent WT, WT + MSO, lf:Yap and lf:Yap + MSO larval lysates respectively, each of which was derived from a pool of 20 larvae; see Supplementary Table 4. ∗∗P < 0.01, two-sided Student’s t-test, values represent the mean ± s.e.m. (c) Proliferation of HepG2 liver cancer cells over 4 days in the presence or absence of glutamine (Q), MSO or VP. n = 3 biologically independent replicates; see Supplementary Table 4. Values represent the mean ± s.e.m.
Supplementary Figure 6 The mTOR pathway is not deregulated by Yap expression or GLUL inhibition, but it is required for Yap-induced hepatomegaly.
(a) Immunohistochemical analysis of phospho-S6 (pS6) levels in liver sections from WT and lf:Yap transgenic fish 24 h after exposure to DMSO, MSO or Rapamycin (RAPA). Scale bars: 50 μm. (b) Analysis of the number of liver cells in H + E stained transverse liver sections from WT and lf:Yap transgenic larvae at 5dpf. n = 16 WT and lf:Yap larvae; see Supplementary Table 4. ∗∗∗P < 0.001, two-sided Student’s t-test, values represent the mean ± s.e.m. (c) Analysis of liver area in H + E stained transverse liver sections from WT and lf:Yap transgenic larvae at 5dpf. n = 16 WT and lf:Yap larvae; see Supplementary Table 4. ∗∗∗P < 0.001, two-sided Student’s t-test, values represent the mean ± s.e.m. (d) Analysis of cellularity (cells/unit2) in H + E stained transverse liver sections from WT and lf:Yap transgenic larvae at 5dpf. n = 16 WT and lf:Yap larvae; see Supplementary Table 4. Values represent the mean ± s.e.m.
Supplementary Figure 7 Yap reprograms the relative isotopic enrichment of nutritional nitrogen into nucleotide biosynthesis in a GLUL-dependent manner to support liver growth.
(a) Relative isotopic enrichment of 15N into deoxyguanosine isotopologues derived from hydrolyzed genomic DNA of liver and 15N-spirulina as determined by LC-MS/MS. One experiment is shown. (b) Histological analysis of cell death (TUNEL) from WT and lf:Yap adults derived from the long-term MSO intervention studies. The positive control showing DAB-stained nuclei is derived from a murine liver section. Scale bar, 50 μm.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3395 kb)
Supplementary Table 1
Supplementary Information (XLSX 35 kb)
Supplementary Table 2
Supplementary Information (XLSX 32 kb)
Supplementary Table 3
Supplementary Information (XLSX 43 kb)
Supplementary Table 4
Supplementary Information (XLSX 137 kb)
Rights and permissions
About this article
Cite this article
Cox, A., Hwang, K., Brown, K. et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol 18, 886–896 (2016). https://doi.org/10.1038/ncb3389
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3389
This article is cited by
-
YAP governs cellular adaptation to perturbation of glutamine metabolism by regulating ATF4-mediated stress response
Oncogene (2023)
-
Amino acid catabolite markers for early prognostication of pneumonia in patients with COVID-19
Nature Communications (2023)
-
The ferroptosis signature predicts the prognosis and immune microenvironment of nasopharyngeal carcinoma
Scientific Reports (2023)
-
Extracellular-matrix mechanics regulate cellular metabolism: A ninja warrior behind mechano-chemo signaling crosstalk
Reviews in Endocrine and Metabolic Disorders (2023)
-
Intratumoral delivery of a Tim-3 antibody-encoding oncolytic adenovirus engages an effective antitumor immune response in liver cancer
Journal of Cancer Research and Clinical Oncology (2023)